Genetic factors for susceptibility to and manifestations of neuromyelitis optica
- PMID: 32979043
- PMCID: PMC7664265
- DOI: 10.1002/acn3.51147
Genetic factors for susceptibility to and manifestations of neuromyelitis optica
Abstract
Objective: To identify genetic factors associated with susceptibility to and clinical features of neuromyelitis optica spectrum disorders (NMOSD).
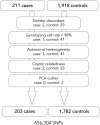
Methods: Genome-wide single nucleotide polymorphism (SNP) genotyping was conducted in 211 Japanese patients with NMOSD fulfilling the 2006 criteria with or without anti-aquaporin-4 (AQP4) antibody and 1,919 Japanese healthy controls (HCs). HLA-DRB1 and HLA-DPB1 alleles were genotyped in 184 NMOSD cases and 317 HCs. Multiple sclerosis (MS) risk alleles outside the major histocompatibility complex (MHC) region were tested in NMOSD and MS genetic burden (MSGB) scores were compared between HCs and NMOSD.
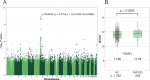
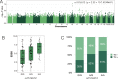
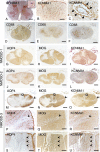
Results: A SNP (rs1964995) in the MHC region was associated with NMOSD susceptibility (odds ratio (OR) = 2.33, P = 4.07 × 10-11 ). HLA-DRB1*08:02 (OR = 2.86, P = 3.03 × 10-4 ) and HLA-DRB1*16:02 (OR = 8.39, P = 1.92 × 10-3 ) were risk alleles for NMOSD susceptibility whereas HLA-DRB1*09:01 was protective (OR = 0.27, P = 1.06 × 10-5 ). Three MS risk variants were associated with susceptibility and MSGB scores were significantly higher in NMOSD than in HCs (P = 0.0095). A SNP in the KCNMA1 (potassium calcium-activated channel subfamily M alpha 1) gene was associated with disability score with genome-wide significance (rs1516512, P = 2.33 × 10-8 ) and transverse myelitis (OR = 1.77, P = 0.011). KCNMA1 was immunohistochemically detected in the perivascular endfeet of astrocytes and its immunoreactivity was markedly diminished in active spinal cord lesions in NMOSD.
Interpretation: Specific HLA-DRB1 alleles confer NMOSD susceptibility and KCNMA1 is associated with disability and transverse myelitis in NMOSD.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Dr. Matsushita received speech honoraria payments from Biogen Japan, Takeda Pharmaceutical Company. Dr. Isobe received grant support from Mitsubishi Tanabe Pharma, Osoegawa Neurology Clinic, Bayer Yakuhin Ltd., and the Japan Blood Products Organization. Dr. Nakamura received grant support from Mitsubishi Tanabe Pharma, Bayer Yakuhin Ltd. and the Japan Blood Products Organization. Dr. Kira received grants and personal fees from Biogen Japan, Bayer Healthcare, Novartis Pharma, Mitsubishi Tanabe Pharma, Eisai, Sanofi, Nobelpharma, Otsuka Pharmaceutical, Chugai Pharmaceutical Company, and Teijin Pharma. No other disclosures were reported.
Figures
Similar articles
-
HLA genotype-clinical phenotype correlations in multiple sclerosis and neuromyelitis optica spectrum disorders based on Japan MS/NMOSD Biobank data.Sci Rep. 2021 Jan 12;11(1):607. doi: 10.1038/s41598-020-79833-7. Sci Rep. 2021. PMID: 33436735 Free PMC article.
-
Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status.J Neurol Neurosurg Psychiatry. 2013 Jan;84(1):29-34. doi: 10.1136/jnnp-2012-302925. Epub 2012 Oct 4. J Neurol Neurosurg Psychiatry. 2013. PMID: 23038741
-
HLA Association With AQP4-IgG-Positive Neuromyelitis Optica Spectrum Disorder in the Korean Population.Neurol Neuroimmunol Neuroinflamm. 2025 May;12(3):e200366. doi: 10.1212/NXI.0000000000200366. Epub 2025 Feb 28. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 40020215 Free PMC article.
-
Neuromyelitis optica is an HLA associated disease different from Multiple Sclerosis: a systematic review with meta-analysis.Sci Rep. 2021 Jan 8;11(1):152. doi: 10.1038/s41598-020-80535-3. Sci Rep. 2021. PMID: 33420337 Free PMC article.
-
Familial neuromyelitis optica spectrum disorders: Case series and systematic review.Mult Scler Relat Disord. 2023 May;73:104627. doi: 10.1016/j.msard.2023.104627. Epub 2023 Mar 21. Mult Scler Relat Disord. 2023. PMID: 37015139
Cited by
-
Methyl-CpG-Binding Protein 2 Emerges as a Central Player in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders.Cell Mol Neurobiol. 2023 Nov;43(8):4071-4101. doi: 10.1007/s10571-023-01432-7. Epub 2023 Nov 13. Cell Mol Neurobiol. 2023. PMID: 37955798 Free PMC article. Review.
-
Neuromyelitis Optica: Pathogenesis Overlap with Other Autoimmune Diseases.Curr Allergy Asthma Rep. 2023 Nov;23(11):647-654. doi: 10.1007/s11882-023-01112-y. Epub 2023 Oct 27. Curr Allergy Asthma Rep. 2023. PMID: 37889429 Review.
-
A national case-control study investigating demographic and environmental factors associated with NMOSD.Mult Scler. 2023 Apr;29(4-5):521-529. doi: 10.1177/13524585231151953. Epub 2023 Feb 18. Mult Scler. 2023. PMID: 36803237 Free PMC article.
-
Advances in biomarkers for optic neuritis and neuromyelitis optica spectrum disorders: a multi-omics perspective.Front Neurol. 2025 May 6;16:1559172. doi: 10.3389/fneur.2025.1559172. eCollection 2025. Front Neurol. 2025. PMID: 40395766 Free PMC article. Review.
-
HLA-transgenic mouse models to study autoimmune central nervous system diseases.Autoimmunity. 2024 Dec;57(1):2387414. doi: 10.1080/08916934.2024.2387414. Epub 2024 Aug 21. Autoimmunity. 2024. PMID: 39167553 Review.
References
-
- Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin‐4 and myelin‐oligodendrocyte glycoprotein antibodies. JAMA Neurol. 2014;71(3):276 Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

