Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison
- PMID: 32979318
- PMCID: PMC7511171
- DOI: 10.1016/S1473-3099(20)30634-4
Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison
Erratum in
-
Correction to Lancet Infect Dis 2020; 20: 1390-400.Lancet Infect Dis. 2020 Dec;20(12):e298. doi: 10.1016/S1473-3099(20)30881-1. Epub 2020 Nov 25. Lancet Infect Dis. 2020. PMID: 33248043 Free PMC article. No abstract available.
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic in 2020. Testing is crucial for mitigating public health and economic effects. Serology is considered key to population-level surveillance and potentially individual-level risk assessment. However, immunoassay performance has not been compared on large, identical sample sets. We aimed to investigate the performance of four high-throughput commercial SARS-CoV-2 antibody immunoassays and a novel 384-well ELISA.
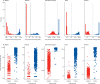
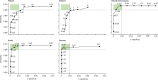
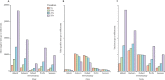
Methods: We did a head-to-head assessment of SARS-CoV-2 IgG assay (Abbott, Chicago, IL, USA), LIAISON SARS-CoV-2 S1/S2 IgG assay (DiaSorin, Saluggia, Italy), Elecsys Anti-SARS-CoV-2 assay (Roche, Basel, Switzerland), SARS-CoV-2 Total assay (Siemens, Munich, Germany), and a novel 384-well ELISA (the Oxford immunoassay). We derived sensitivity and specificity from 976 pre-pandemic blood samples (collected between Sept 4, 2014, and Oct 4, 2016) and 536 blood samples from patients with laboratory-confirmed SARS-CoV-2 infection, collected at least 20 days post symptom onset (collected between Feb 1, 2020, and May 31, 2020). Receiver operating characteristic (ROC) curves were used to assess assay thresholds.
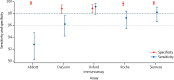
Findings: At the manufacturers' thresholds, for the Abbott assay sensitivity was 92·7% (95% CI 90·2-94·8) and specificity was 99·9% (99·4-100%); for the DiaSorin assay sensitivity was 96·2% (94·2-97·7) and specificity was 98·9% (98·0-99·4); for the Oxford immunoassay sensitivity was 99·1% (97·8-99·7) and specificity was 99·0% (98·1-99·5); for the Roche assay sensitivity was 97·2% (95·4-98·4) and specificity was 99·8% (99·3-100); and for the Siemens assay sensitivity was 98·1% (96·6-99·1) and specificity was 99·9% (99·4-100%). All assays achieved a sensitivity of at least 98% with thresholds optimised to achieve a specificity of at least 98% on samples taken 30 days or more post symptom onset.
Interpretation: Four commercial, widely available assays and a scalable 384-well ELISA can be used for SARS-CoV-2 serological testing to achieve sensitivity and specificity of at least 98%. The Siemens assay and Oxford immunoassay achieved these metrics without further optimisation. This benchmark study in immunoassay assessment should enable refinements of testing strategies and the best use of serological testing resource to benefit individuals and population health.
Funding: Public Health England and UK National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Immunoassays for anti-SARS-CoV-2 antibodies: recent insights.Lancet Infect Dis. 2021 May;21(5):e120. doi: 10.1016/S1473-3099(20)30846-X. Epub 2020 Oct 30. Lancet Infect Dis. 2021. PMID: 33137289 Free PMC article. No abstract available.
References
-
- Center for Systems and Science Engineering at Johns Hopkins University COVID-19 dashboard. 2020. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

