Prolonged Tracheal Intubation and the Association Between Patent Ductus Arteriosus and Bronchopulmonary Dysplasia: A Secondary Analysis of the PDA-TOLERATE trial
- PMID: 32979387
- PMCID: PMC7855529
- DOI: 10.1016/j.jpeds.2020.09.047
Prolonged Tracheal Intubation and the Association Between Patent Ductus Arteriosus and Bronchopulmonary Dysplasia: A Secondary Analysis of the PDA-TOLERATE trial
Abstract
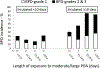
In the PDA-TOLERATE trial, persistent (even for several weeks) moderate to large patent ductus arteriosus (PDA) was not associated with an increased risk of BPD when the infant required <10 days of intubation. However, in infants requiring intubation for ≥10 days, prolonged PDA exposure (≥11 days) was associated with an increased risk of moderate/severe BPD.
Keywords: bronchopulmonary dysplasia; patent ductus arteriosus; premature birth.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Prolonged Ductal Patency in Preterm Infants: Does It Matter?J Pediatr. 2021 Feb;229:12-14.e1. doi: 10.1016/j.jpeds.2020.10.059. Epub 2020 Oct 30. J Pediatr. 2021. PMID: 33130156 No abstract available.
References
-
- Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory Closure Versus Nonintervention for Patent Ductus Arteriosus in Very Preterm Infants. J Pediatr. 2016;177:66–71 - PubMed
-
- Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99:F99–F104. - PubMed
-
- Aranda JV, Clyman R, Cox B, Van Overmeire B, Wozniak P, Sosenko I, et al. A randomized, double-blind, placebo-controlled trial on intravenous ibuprofen L-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 hours of birth in extremely low-birth-weight infants. Am J Perinatol. 2009;26:235–45. - PubMed
-
- Al Faleh K, Smyth J, Roberts R, Solimano A, Asztalos E, Schmidt B. Prevention and 18-month outcome of serious pulmonary hemorrhage in extremely low birth weight infants: results from the triall of indomethacin prophylaxis in preterms. Pediatrics. 2008;121:e233–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

