Determination of SARS-CoV-2 antibodies with assays from Diasorin, Roche and IDvet
- PMID: 32979407
- PMCID: PMC7510775
- DOI: 10.1016/j.jviromet.2020.113978
Determination of SARS-CoV-2 antibodies with assays from Diasorin, Roche and IDvet
Abstract
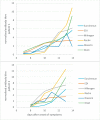
There is an ongoing need for highly reliable serological assays to detect individuals with past SARS-CoV-2 infection. Using 75 sera from patients tested positive or negative by SARS-CoV-2 PCR, we investigated the sensitivity and specificity of the Liaison SARS-CoV-2 S1/S2 IgG assay (DiaSorin), the Elecsys Anti-SARS-CoV-2 assay (Roche), and the ID Screen SARS-CoV-2-N IgG indirect kit (IDVet). We determined a sensitivity of 95.5 %, 95.5 %, and 100 % and a specificity of 90.5 %, 96.2 %, and 92.5 % for the DiaSorin assay, the Roche assay, and the IDVet assay, respectively. We conclude that serologic assays combining very high sensitivity and specificity are still not commercially available for SARS-CoV-2. For maximizing sensitivity and specificity of SARS-CoV-2 serological diagnostics, the combination of two assays may be helpful.
Keywords: COVID-19; ELISA; SARS-CoV-2; Serology.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
We declare no conflict of interest
Figures
References
-
- Eis-Hübinger A.M., Hönemann M., Wenzel J.J., Berger A., Widera M., Schmidt B., Aldabbagh S., Marx B., Streeck H., Ciesek S., Liebert U.G., Huzly D., Hengel H., Panning M. Ad hoc laboratory-based surveillance of SARS-CoV-2 by real-time RT-PCR using minipools of RNA prepared from routine respiratory samples. J. Clin. Virol. 2020;127 - PMC - PubMed
-
- Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020;580:571–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous