Assessing the Resident Progenitor Cell Population and the Vascularity of the Adult Human Meniscus
- PMID: 32979500
- PMCID: PMC7829352
- DOI: 10.1016/j.arthro.2020.09.021
Assessing the Resident Progenitor Cell Population and the Vascularity of the Adult Human Meniscus
Abstract
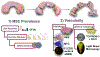
Purpose: To identify, characterize, and compare the resident progenitor cell populations within the red-red, red-white, and white-white (WW) zones of freshly harvested human cadaver menisci and to characterize the vascularity of human menisci using immunofluorescence and 3-dimensional (3D) imaging.
Methods: Fresh adult human menisci were harvested from healthy donors. Menisci were enzymatically digested, mononuclear cells isolated, and characterized using flow cytometry with antibodies against mesenchymal stem cell surface markers (CD105, CD90, CD44, and CD29). Cells were expanded in culture, characterized, and compared with bone marrow-derived mesenchymal stem cells. Trilineage differentiation potential of cultured cells was determined. Vasculature of menisci was mapped in 3D using a modified uDisco clearing and immunofluorescence against vascular markers CD31, lectin, and alpha smooth muscle actin.
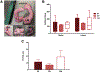
Results: There were no significant differences in the clonogenicity of isolated cells between the 3 zones. Flow cytometry showed presence of CD44+CD105+CD29+CD90+ cells in all 3 zones with high prevalence in the WW zone. Progenitors from all zones were found to be potent to differentiate to mesenchymal lineages. Larger vessels in the red-red zone of meniscus were observed spanning toward red-white, sprouting to smaller arterioles and venules. CD31+ cells were identified in all zones using the 3D imaging and co-localization of additional markers of vasculature (lectin and alpha smooth muscle actin) was observed.
Conclusions: The presence of resident mesenchymal progenitors was evident in all 3 meniscal zones of healthy adult donors without injury. In addition, our results demonstrate the presence of vascularization in the WW zone.
Clinical relevance: The existence of progenitors and presence of microvasculature in the WW zone of the meniscus suggests the potential for repair and biologic augmentation strategies in that zone of the meniscus in young healthy adults. Further research is necessary to fully define the functionality of the meniscal blood supply and its implications for repair.
Copyright © 2020 Arthroscopy Association of North America. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Editorial Commentary: Discovery: Progenitor Cells and Endothelial Cells Are Found in the White-White Zone of the Meniscus, But This Does Not Mean That These Tears Heal or Should Be Repaired.Arthroscopy. 2021 Jan;37(1):266-267. doi: 10.1016/j.arthro.2020.11.007. Arthroscopy. 2021. PMID: 33384087
Similar articles
-
Identification and characterization of adult mouse meniscus stem/progenitor cells.Connect Tissue Res. 2017 May-Jul;58(3-4):238-245. doi: 10.1080/03008207.2016.1271797. Epub 2016 Dec 22. Connect Tissue Res. 2017. PMID: 28005443
-
Relevance of meniscal cell regional phenotype to tissue engineering.Connect Tissue Res. 2017 May-Jul;58(3-4):259-270. doi: 10.1080/03008207.2016.1268604. Epub 2016 Dec 7. Connect Tissue Res. 2017. PMID: 27925477 Free PMC article.
-
Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors.Acta Biomater. 2018 Oct 15;80:131-143. doi: 10.1016/j.actbio.2018.09.038. Epub 2018 Sep 26. Acta Biomater. 2018. PMID: 30267878
-
Age-Related Changes in the Microvascular Density of the Human Meniscus.Am J Sports Med. 2021 Nov;49(13):3544-3550. doi: 10.1177/03635465211039865. Epub 2021 Sep 30. Am J Sports Med. 2021. PMID: 34591716
-
The clinical potential of meniscal progenitor cells.J Cartil Jt Preserv. 2024 Dec;4(4):None. doi: 10.1016/j.jcjp.2024.100166. J Cartil Jt Preserv. 2024. PMID: 39669533 Free PMC article. Review.
Cited by
-
Fibronectin Adherent Cell Populations Derived From Avascular and Vascular Regions of the Meniscus Have Enhanced Clonogenicity and Differentiation Potential Under Physioxia.Front Bioeng Biotechnol. 2022 Jan 28;9:789621. doi: 10.3389/fbioe.2021.789621. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35155405 Free PMC article.
-
Indocyanine Green Fluorescence-Guided Knee Arthroscopy: A Technical Note for Investigating the Microvasculature Around the Meniscus.Arthrosc Tech. 2024 Jan 1;13(3):102878. doi: 10.1016/j.eats.2023.11.006. eCollection 2024 Mar. Arthrosc Tech. 2024. PMID: 38584628 Free PMC article.
-
Directing iPSC differentiation into iTenocytes using combined scleraxis overexpression and cyclic loading.J Orthop Res. 2023 Jun;41(6):1148-1161. doi: 10.1002/jor.25459. Epub 2022 Oct 26. J Orthop Res. 2023. PMID: 36203346 Free PMC article.
-
Selection of Highly Proliferative and Multipotent Meniscus Progenitors through Differential Adhesion to Fibronectin: A Novel Approach in Meniscus Tissue Engineering.Int J Mol Sci. 2021 Aug 10;22(16):8614. doi: 10.3390/ijms22168614. Int J Mol Sci. 2021. PMID: 34445320 Free PMC article.
-
Meniscal repair: The current state and recent advances in augmentation.J Orthop Res. 2021 Jul;39(7):1368-1382. doi: 10.1002/jor.25021. Epub 2021 Mar 19. J Orthop Res. 2021. PMID: 33751642 Free PMC article.
References
-
- Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med 1983;11:131–141. - PubMed
-
- Chahla J, Cinque ME, Godin JA, et al. Meniscectomy and resultant articular cartilage lesions of the knee among prospective National Football League players: An imaging and performance analysis. Am J Sports Med 2018;46: 200–207. - PubMed
-
- Dave LY, Caborn DN. Outside-in meniscus repair: The last 25 years. Sports Med Arthrosc 2012;20:77–85. - PubMed
-
- Stein T, Mehling AP, Welsch F, von Eisenhart-Rothe R, Jäger A. Long-term outcome after arthroscopic meniscal repair versus arthroscopic partial meniscectomy for traumatic meniscal tears. American J Sports Med 2010;38: 1542–1548. - PubMed
-
- Rodeo SA, Monibi F, Dehghani B, Maher S. Biological and mechanical predictors of meniscus function: Basic science to clinical translation. J Orthop Res 2020;38:937–945. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

