Oxylipins in plastidial retrograde signaling
- PMID: 32979794
- PMCID: PMC7511966
- DOI: 10.1016/j.redox.2020.101717
Oxylipins in plastidial retrograde signaling
Abstract
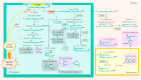
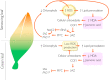
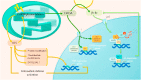
Oxylipins (compounds derived from the oxidation of polyunsaturated fatty acids) are essential in retrograde signaling emanating from plastids to the nucleus during plant developmental and stress responses. In this graphical review, we provide an overview of the chemical structure, biosynthesis and role of oxylipins, as both redox and hormonal signals, in controlling plant development and stress responses. We also briefly summarize current gaps in the understanding of the involvement of oxylipins in plastidial retrograde signaling to highlight future avenues for research.
Keywords: Chloroplasts; Jasmonates; Oxylipins; Plastids; ROS; Redox signaling.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The plastidial retrograde signal methyl erythritol cyclopyrophosphate is a regulator of salicylic acid and jasmonic acid crosstalk.J Exp Bot. 2016 Mar;67(5):1557-66. doi: 10.1093/jxb/erv550. Epub 2016 Jan 4. J Exp Bot. 2016. PMID: 26733689 Free PMC article.
-
Oxylipins: structurally diverse metabolites from fatty acid oxidation.Plant Physiol Biochem. 2009 Jun;47(6):511-7. doi: 10.1016/j.plaphy.2008.12.011. Epub 2008 Dec 25. Plant Physiol Biochem. 2009. PMID: 19167233 Review.
-
Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant.Plant Cell. 2003 Dec;15(12):2952-65. doi: 10.1105/tpc.017301. Epub 2003 Nov 13. Plant Cell. 2003. PMID: 14615603 Free PMC article.
-
Impact of phyto-oxylipins in plant defense.Trends Plant Sci. 2002 Jul;7(7):315-22. doi: 10.1016/s1360-1385(02)02290-2. Trends Plant Sci. 2002. PMID: 12119169 Review.
-
Impact of cyclopentenone-oxylipins on the proteome of Arabidopsis thaliana.Biochim Biophys Acta. 2008 Dec;1784(12):1975-85. doi: 10.1016/j.bbapap.2008.09.003. Epub 2008 Sep 20. Biochim Biophys Acta. 2008. PMID: 18848650
Cited by
-
Transcriptomic and metabolic signatures of diatom plasticity to light fluctuations.Plant Physiol. 2022 Nov 28;190(4):2295-2314. doi: 10.1093/plphys/kiac455. Plant Physiol. 2022. PMID: 36149329 Free PMC article.
-
Ethylene and Jasmonates Signaling Network Mediating Secondary Metabolites under Abiotic Stress.Int J Mol Sci. 2023 Mar 22;24(6):5990. doi: 10.3390/ijms24065990. Int J Mol Sci. 2023. PMID: 36983071 Free PMC article. Review.
-
Vision, challenges and opportunities for a Plant Cell Atlas.Elife. 2021 Sep 7;10:e66877. doi: 10.7554/eLife.66877. Elife. 2021. PMID: 34491200 Free PMC article.
-
Linking jasmonates with vitamin E accumulation in plants: a case study in the Mediterranean shrub Cistus albidus L.Planta. 2021 Jan 18;253(2):36. doi: 10.1007/s00425-021-03570-y. Planta. 2021. PMID: 33462640
-
Stressing the importance of plant specialized metabolites: omics-based approaches for discovering specialized metabolism in plant stress responses.Front Plant Sci. 2023 Nov 8;14:1272363. doi: 10.3389/fpls.2023.1272363. eCollection 2023. Front Plant Sci. 2023. PMID: 38023861 Free PMC article. Review.
References
-
- Farmer E.E., Mueller M.J. ROS-mediated lipid leroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013;64:429–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

