Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation
- PMID: 32979838
- PMCID: PMC7519249
- DOI: 10.1016/j.ebiom.2020.103014
Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation
Abstract
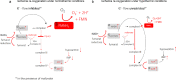
Background: Mitochondrial succinate accumulation has been suggested as key event for ischemia reperfusion injury in mice. No specific data are however available on behavior of liver mitochondria during ex situ machine perfusion in clinical transplant models.
Methods: We investigated mitochondrial metabolism of isolated perfused rat livers before transplantation. Livers were exposed to warm and cold ischemia to simulate donation after circulatory death (DCD) and organ transport. Subsequently, livers were perfused with oxygenated Belzer-MPS for 1h, at hypothermic or normothermic conditions. Various experiments were performed with supplemented succinate and/or mitochondrial inhibitors. The perfusate, liver tissues, and isolated mitochondria were analyzed by mass-spectroscopy and fluorimetry. Additionally, rat DCD livers were transplanted after 1h hypothermic or normothermic oxygenated perfusion. In parallel, perfusate samples were analysed during HOPE-treatment of human DCD livers before transplantation.
Findings: Succinate exposure during rat liver perfusion triggered a dose-dependent release of mitochondrial Flavin-Mononucleotide (FMN) and NADH in perfusates under normothermic conditions. In contrast, perfusate FMN was 3-8 fold lower under hypothermic conditions, suggesting less mitochondrial injury during cold re-oxygenation compared to normothermic conditions. HOPE-treatment induced a mitochondrial reprogramming with uploading of the nucleotide pool and effective succinate metabolism. This resulted in a clear superiority after liver transplantation compared to normothermic perfusion. Finally, the degree of mitochondrial injury during HOPE of human DCD livers, quantified by perfusate FMN and NADH, was predictive for liver function.
Interpretation: Mitochondrial injury determines outcome of transplanted rodent and human livers. Hypothermic oxygenated perfusion improves mitochondrial function, and allows viability assessment of liver grafts before implantation.
Funding: detailed information can be found in Acknowledgments.
Keywords: Complex I; FMN; Hypothermic oxygenated perfusion; Liver transplantation; Normothermic oxygenated perfusion.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Interests This study was conducted at the University Hospital Zurich (USZ). Raw data and laboratory analysis results were extracted at the laboratory of the Department of Visceral Surgery and Transplantation of the USZ. Data analysis was carried out at the laboratory of the Department of Visceral Surgery and Transplantation of the USZ. The authors have declared that no competing interests exist. The study was principally funded by a main research grant from the swiss national foundation (SNF), awarded to Professor Philipp Dutkowski (Ref: 32003B-140776/1, 3200B-153012/1, 31IC30-166909). Mitochondrial and tissue analysis was further supported by the Max Planck Society (Dr. David Meierhofer) and the NIH grant: R01NS112381 awarded to Dr. Alexander Galkin. There are no patents involved or affecting this study.
Figures








References
-
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J [Internet] 2009;417(1):1–13. http://www.ncbi.nlm.nih.gov/pubmed/19061483%5Cnhttp://www.pubmedcentral.... Available from: - DOI - PMC - PubMed
-
- Van Raemdonck D, Neyrinck A, Rega F, Devos T, Pirenne J. Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transpl. 2013;18(1):24–33. - PubMed
-
- Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL. A randomized trial of normothermic preservation in liver transplantation. Nature [Internet] 2018 http://www.nature.com/articles/s41586-018-0047-9 Available from: - PubMed

