Advances in extracorporeal membrane oxygenator design for artificial placenta technology
- PMID: 32979857
- PMCID: PMC8513573
- DOI: 10.1111/aor.13827
Advances in extracorporeal membrane oxygenator design for artificial placenta technology
Abstract
Extreme prematurity, defined as a gestational age of fewer than 28 weeks, is a significant health problem worldwide. It carries a high burden of mortality and morbidity, in large part due to the immaturity of the lungs at this stage of development. The standard of care for these patients includes support with mechanical ventilation, which exacerbates lung pathology. Extracorporeal life support (ECLS), also called artificial placenta technology when applied to extremely preterm (EPT) infants, offers an intriguing solution. ECLS involves providing gas exchange via an extracorporeal device, thereby doing the work of the lungs and allowing them to develop without being subjected to injurious mechanical ventilation. While ECLS has been successfully used in respiratory failure in full-term neonates, children, and adults, it has not been applied effectively to the EPT patient population. In this review, we discuss the unique aspects of EPT infants and the challenges of applying ECLS to these patients. In addition, we review recent progress in artificial placenta technology development. We then offer analysis on design considerations for successful engineering of a membrane oxygenator for an artificial placenta circuit. Finally, we examine next-generation oxygenators that might advance the development of artificial placenta devices.
Keywords: artificial placenta; extracorporeal life support; extreme prematurity; hollow-fiber membranes; microfluidics.
© 2020 International Center for Artificial Organs and Transplantation and Wiley Periodicals LLC.
Conflict of interest statement
Conflicts of Interest:
The authors report no conflicts of interest.
Figures

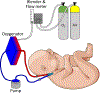

References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. The Lancet. 2012; - PubMed
-
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final data for 2017. National Vital Statistics Reports. 2018. November;67(8):1–49. - PubMed
-
- Whitsett JA, Wert SE. Molecular Determinants of Lung Morphogenesis. In: Chernick V, Wilmott RW, Boat TF, Bush A, editors. Kendig’s Disorders of the Respiratory Tract in Children. 7th ed. Philadelphia, PA: Saunders Elsevier; 2006.

