The actin polymerization factor Diaphanous and the actin severing protein Flightless I collaborate to regulate sarcomere size
- PMID: 32980309
- PMCID: PMC8279456
- DOI: 10.1016/j.ydbio.2020.09.014
The actin polymerization factor Diaphanous and the actin severing protein Flightless I collaborate to regulate sarcomere size
Abstract
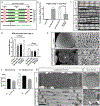
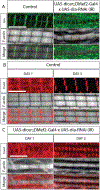
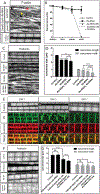
The sarcomere is the basic contractile unit of muscle, composed of repeated sets of actin thin filaments and myosin thick filaments. During muscle development, sarcomeres grow in size to accommodate the growth and function of muscle fibers. Failure in regulating sarcomere size results in muscle dysfunction; yet, it is unclear how the size and uniformity of sarcomeres are controlled. Here we show that the formin Diaphanous is critical for the growth and maintenance of sarcomere size: Dia sets sarcomere length and width through regulation of the number and length of the actin thin filaments in the Drosophila flight muscle. To regulate thin filament length and sarcomere size, Dia interacts with the Gelsolin superfamily member Flightless I (FliI). We suggest that these actin regulators, by controlling actin dynamics and turnover, generate uniformly sized sarcomeres tuned for the muscle contractions required for flight.
Keywords: Actin filaments; Actin polymerization; Actin severing; Diaphanous; Drosophila; Flight muscle; Flightless I; Formins; Gelsolin; Muscle maintenance; Sarcomere.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures


References
-
- Afshar K, Stuart B, Wasserman SA, 2000. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 127, 1887–1897. - PubMed
-
- Arimura T, Takeya R, Ishikawa T, Yamano T, Matsuo A, Tatsumi T, Nomura T, Sumimoto H, Kimura A, 2013. Dilated cardiomyopathy-associated FHOD3 variant impairs the ability to induce activation of transcription factor serum response factor. Circ J 77, 2990–2996. - PubMed
-
- Bai J, Hartwig JH, Perrimon N, 2007. SALS, a WH2-domain-containing protein, promotes sarcomeric actin filament elongation from pointed ends during Drosophila muscle growth. Dev Cell 13, 828–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

