Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration Using Deep Learning
- PMID: 32980396
- PMCID: PMC8528155
- DOI: 10.1016/j.ophtha.2020.09.025
Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration Using Deep Learning
Abstract
Purpose: To apply a deep learning algorithm for automated, objective, and comprehensive quantification of OCT scans to a large real-world dataset of eyes with neovascular age-related macular degeneration (AMD) and make the raw segmentation output data openly available for further research.
Design: Retrospective analysis of OCT images from the Moorfields Eye Hospital AMD Database.
Participants: A total of 2473 first-treated eyes and 493 second-treated eyes that commenced therapy for neovascular AMD between June 2012 and June 2017.
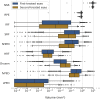
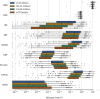
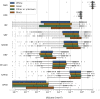
Methods: A deep learning algorithm was used to segment all baseline OCT scans. Volumes were calculated for segmented features such as neurosensory retina (NSR), drusen, intraretinal fluid (IRF), subretinal fluid (SRF), subretinal hyperreflective material (SHRM), retinal pigment epithelium (RPE), hyperreflective foci (HRF), fibrovascular pigment epithelium detachment (fvPED), and serous PED (sPED). Analyses included comparisons between first- and second-treated eyes by visual acuity (VA) and race/ethnicity and correlations between volumes.
Main outcome measures: Volumes of segmented features (mm3) and central subfield thickness (CST) (μm).
Results: In first-treated eyes, the majority had both IRF and SRF (54.7%). First-treated eyes had greater volumes for all segmented tissues, with the exception of drusen, which was greater in second-treated eyes. In first-treated eyes, older age was associated with lower volumes for RPE, SRF, NSR, and sPED; in second-treated eyes, older age was associated with lower volumes of NSR, RPE, sPED, fvPED, and SRF. Eyes from Black individuals had higher SRF, RPE, and serous PED volumes compared with other ethnic groups. Greater volumes of the majority of features were associated with worse VA.
Conclusions: We report the results of large-scale automated quantification of a novel range of baseline features in neovascular AMD. Major differences between first- and second-treated eyes, with increasing age, and between ethnicities are highlighted. In the coming years, enhanced, automated OCT segmentation may assist personalization of real-world care and the detection of novel structure-function correlations. These data will be made publicly available for replication and future investigation by the AMD research community.
Keywords: OCT; age-related macular degeneration; automated; deep learning; neovascular.
Copyright © 2020 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Schmidt-Erfurth U., Waldstein S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. - PubMed
-
- Waldstein S.M., Philip A.-M., Leitner R. Correlation of 3-dimensionally quantified intraretinal and subretinal fluid with visual acuity in neovascular age-related macular degeneration. JAMA Ophthalmol. 2016;134:182–190. - PubMed
-
- Fung A.E., Lalwani G.A., Rosenfeld P.J. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. - PubMed
-
- Lalwani G.A., Rosenfeld P.J., Fung A.E. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58.e1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

