Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval
- PMID: 32980449
- PMCID: PMC8173698
- DOI: 10.1016/j.addr.2020.09.009
Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval
Abstract
Over the last three decades, polymeric micelles have emerged as a highly promising drug delivery platform for therapeutic compounds. Particularly, poorly soluble small molecules with high potency and significant toxicity were encapsulated in polymeric micelles. Polymeric micelles have shown improved pharmacokinetic profiles in preclinical animal models and enhanced efficacy with a superior safety profile for therapeutic drugs. Several polymeric micelle formulations have reached the clinical stage and are either in clinical trials or are approved for human use. This furthers interest in this field and underscores the need for additional learning of how to best design and apply these micellar carriers to improve the clinical outcomes of many drugs. In this review, we provide detailed information on polymeric micelles for the solubilization of poorly soluble small molecules in topics such as the design of block copolymers, experimental and theoretical analysis of drug encapsulation in polymeric micelles, pharmacokinetics of drugs in polymeric micelles, regulatory approval pathways of nanomedicines, and current outcomes from micelle formulations in clinical trials. We aim to describe the latest information on advanced analytical approaches for elucidating molecular interactions within the core of polymeric micelles for effective solubilization as well as for analyzing nanomedicine's pharmacokinetic profiles. Taking into account the considerations described within, academic and industrial researchers can continue to elucidate novel interactions in polymeric micelles and capitalize on their potential as drug delivery vehicles to help improve therapeutic outcomes in systemic delivery.
Keywords: Active pharmaceutical ingredient; Block copolymer; Clinical trials; Drug; Drug delivery; Polymeric micelle; Solubilization.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Kabanov is the co-developer of SP1049C and has interest in SoftKemo. He is also a co-founder and interested in the commercial success of DelAqua Pharmaceuticals Inc. which has the intent of developing of polymeric micelle drug formulations. Kabanov is co-inventor on US Patent 9.402,908B2 pertinent to the subject matter. The other authors have no competing interests to report.
Figures

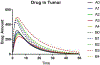

References
-
- Bharate SS, Bharate SB, Bajaj A, Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review, J. excip. food chem, 1 (2016) 1131.
-
- Cabral H, Miyata K, Osada K, Kataoka K, Block Copolymer Micelles in Nanomedicine Applications, Chem. Rev, 118 (2018) 6844–6892. - PubMed
-
- Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S, Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly (ethylene glycol)-poly (aspartic acid) block copolymer, Cancer Res, 50 (1990) 1693–1700. - PubMed
-
- Kabanov AV, Chekhonin V, Alakhov VY, Batrakova E, Lebedev A, Melik-Nubarov N, Arzhakov S, Levashov A, Morozov G, Severin E, The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles: micelles as microcontainers for drug targeting, FEBS Lett, 258 (1989) 343–345. - PubMed
-
- Kwon GS, Kataoka K, Block copolymer micelles as long-circulating drug vehicles, Adv. Drug Deliv. Rev, 16 (1995) 295–309.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

