Retinoic acid signalling in fibro/adipogenic progenitors robustly enhances muscle regeneration
- PMID: 32980698
- PMCID: PMC7519288
- DOI: 10.1016/j.ebiom.2020.103020
Retinoic acid signalling in fibro/adipogenic progenitors robustly enhances muscle regeneration
Abstract
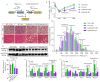
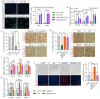
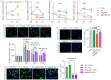
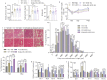
Background: During muscle regeneration, excessive formation of adipogenic and fibrogenic tissues, from their respective fibro/adipogenic progenitors (FAPs), impairs functional recovery. Intrinsic mechanisms controlling the proliferation and differentiation of FAPs remain largely unexplored.
Methods: Here, we investigated the role of retinoic acid (RA) signalling in regulating FAPs and the subsequent effects on muscle restoration from a cardiotoxin-induced injury. Blockage of retinoic acid receptor (RAR) signalling was achieved through dominant negative retinoic acid receptor α (RARα403) expression specific in PDGFRα+ FAPs in vivo and by BMS493 treatment in vitro. Effects of RAR-signalling on FAP cellularity and muscle regeneration were also investigated in a high-fat diet-induced obese mice model.
Findings: Supplementation of RA increased the proliferation of FAPs during the early stages of regeneration while suppressing FAP differentiation and promoting apoptosis during the remodelling stage. Loss of RAR-signalling caused ectopic adipogenic differentiation of FAPs and impaired muscle regeneration. Furthermore, obesity disrupted the cellular transition of FAPs and attenuated muscle regeneration. Supplementation of RA to obese mice not only rescued impaired muscle fibre regeneration, but also inhibited infiltration of fat and fibrotic tissues during muscle repair. These beneficial effects were abolished after blocking RAR-signalling in FAPs of obese mice.
Interpretation: These data suggest that RAR-signalling in FAPs is a critical therapeutic target for suppressing differentiation of FAPs and facilitating the regeneration of muscle and other tissues.
Funding: This study was supported by grants from the National Institutes of Health (R01-HD067449 and R21-AG049976) to M.D.
Keywords: Adipogenesis; Fibro/adipogenic progenitors; Fibrosis; Muscle regeneration; Obesity; Retinoic acid signalling.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of Competing Interests The authors declare no conflict of interest.
Figures


Similar articles
-
Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors.Cell Commun Signal. 2018 Jul 20;16(1):42. doi: 10.1186/s12964-018-0251-0. Cell Commun Signal. 2018. PMID: 30029643 Free PMC article.
-
The Role of Matrix Metalloproteinase-13 (MMP13) in TGFβ/BMP Pathway Regulation of Fibro-Adipogenic Progenitor (FAP) Differentiation.Cell Physiol Biochem. 2022 Dec 20;56(6):730-743. doi: 10.33594/000000596. Cell Physiol Biochem. 2022. PMID: 36537139
-
The role of oxidative stress-mediated fibro-adipogenic progenitor senescence in skeletal muscle regeneration and repair.Stem Cell Res Ther. 2025 Mar 1;16(1):104. doi: 10.1186/s13287-025-04242-4. Stem Cell Res Ther. 2025. PMID: 40025535 Free PMC article.
-
Thrown for a loop: fibro-adipogenic progenitors in skeletal muscle fibrosis.Am J Physiol Cell Physiol. 2023 Oct 1;325(4):C895-C906. doi: 10.1152/ajpcell.00245.2023. Epub 2023 Aug 21. Am J Physiol Cell Physiol. 2023. PMID: 37602412 Free PMC article. Review.
-
Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases.Open Biol. 2021 Dec;11(12):210110. doi: 10.1098/rsob.210110. Epub 2021 Dec 8. Open Biol. 2021. PMID: 34875199 Free PMC article. Review.
Cited by
-
Identification of a KLF5-dependent program and drug development for skeletal muscle atrophy.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2102895118. doi: 10.1073/pnas.2102895118. Proc Natl Acad Sci U S A. 2021. PMID: 34426497 Free PMC article.
-
Fork in the road: therapeutic and pathological actions for fibro-adipogenic progenitors following musculoskeletal injury.J Physiol. 2025 Feb 11:10.1113/JP286816. doi: 10.1113/JP286816. Online ahead of print. J Physiol. 2025. PMID: 39930980 Free PMC article. Review.
-
Oestrogen suppresses the adipogenesis of fibro/adipogenic progenitors through reactivating the METTL3-ESR1-mediated loop in post-menopausal females.Clin Transl Med. 2025 Feb;15(2):e70206. doi: 10.1002/ctm2.70206. Clin Transl Med. 2025. PMID: 39875775 Free PMC article.
-
Vitamin A Promotes the Repair of Mice Skeletal Muscle Injury through RARα.Nutrients. 2023 Aug 22;15(17):3674. doi: 10.3390/nu15173674. Nutrients. 2023. PMID: 37686706 Free PMC article.
-
A 3D adipogenesis platform to study the fate of fibro/adipogenic progenitors in muscular dystrophies.Dis Model Mech. 2023 Jun 1;16(6):dmm049915. doi: 10.1242/dmm.049915. Epub 2023 Jun 23. Dis Model Mech. 2023. PMID: 37272428 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

