Impact of breastfeeding, maternal antiretroviral treatment and health service factors on 18-month vertical transmission of HIV and HIV-free survival: results from a nationally representative HIV-exposed infant cohort, South Africa
- PMID: 32980812
- PMCID: PMC11459440
- DOI: 10.1136/jech-2019-213453
Impact of breastfeeding, maternal antiretroviral treatment and health service factors on 18-month vertical transmission of HIV and HIV-free survival: results from a nationally representative HIV-exposed infant cohort, South Africa
Abstract
Background: We analysed the impact of breastfeeding, antiretroviral drugs and health service factors on cumulative (6 weeks to 18 months) vertical transmission of HIV (MTCT) and 'MTCT-or-death', in South Africa, and compared estimates with global impact criteria to validate MTCT elimination: (1) <5% final MTCT and (2) case rate ≤50 (new paediatric HIV infections/100 000 live births).
Methods: 9120 infants aged 6 weeks were enrolled in a nationally representative survey. Of 2811 HIV-exposed uninfected infants (HEU), 2644 enrolled into follow-up (at 3, 6, 9, 12, 15 and 18 months). Using Kaplan-Meier analysis and weighted survey domain-based Cox proportional hazards models, we estimated cumulative risk of MTCT and 'MTCT or death' and risk factors for time-to-event outcomes, adjusting for study design and loss-to-follow-up.
Results: Cumulative (final) MTCT was 4.3% (95% CI 3.7% to 5.0%); case rate was 1290. Postnatal MTCT (>6 weeks to 18 months) was 1.7% (95% CI 1.2% to 2.4%). Cumulative 'MTCT-or-death' was 6.3% (95% CI 5.5% to 7.3%); 81% and 62% of cumulative MTCT and 'MTCT-or-death', respectively, occurred by 6 months. Postnatal MTCT increased with unknown maternal CD4-cell-count (adjusted HR (aHR 2.66 (1.5-5.6)), undocumented maternal HIV status (aHR 2.21 (1.0-4.7)) and exclusive (aHR 2.3 (1.0-5.2)) or mixed (aHR 3.7 (1.2-11.4)) breastfeeding. Cumulative 'MTCT-or death' increased in households with 'no refrigerator' (aHR 1.7 (1.1-2.9)) and decreased if infants used nevirapine at 6 weeks (aHR 0.4 (0.2-0.9)).
Conclusions: While the <5% final MTCT target was met, the case rate was 25-times above target. Systems are needed in the first 6 months post-delivery to optimise HEU health and fast-track ART initiation in newly diagnosed mothers.
Keywords: Public health; child health; epidemiology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
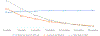

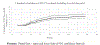
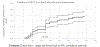
References
-
- Goga AE. Infant feeding and HIV: towards a new implementation plan for minimising postnatal HIV transmission and maximising HIV-free survival. South Afr J HIV Med 2009;10:20. Available https://www.thefreelibrary.com/Infant+feeding+and+HIV%3a+towards+a+new+p... (accessed 1 Nov 2019)
-
- Kumwenda NHD, Mofenson L, Thigpen M, et al. Extended antiretroviral prophylaxis to reduce breastmilk HIV-1 transmission. N Engl J Med 2008;359:119–29. - PubMed
-
- Six week extended dose nevirapine (SWEN) Study team, et al. Extended dose nevirapine at 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India and Uganda: an analysis of 3 randomised controlled trials. Lancet 2008;372:300–13. - PubMed
-
- World Health Organisation. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Apr 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
