Blood and marrow transplantation during the emerging COVID-19 pandemic: the Seattle approach
- PMID: 32980860
- PMCID: PMC7519858
- DOI: 10.1038/s41409-020-01068-x
Blood and marrow transplantation during the emerging COVID-19 pandemic: the Seattle approach
Abstract
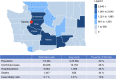
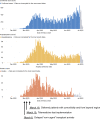
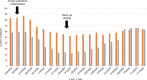
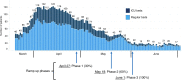
On January 20, 2020, the first patient with coronavirus disease 2019 (COVID-19) in the United States of America was diagnosed in Washington state, which subsequently experienced rapidly increasing numbers of COVID-19 cases, hospitalizations, and deaths. This placed the Seattle Blood and Marrow Transplant Program at Fred Hutchinson Cancer Research Center (Fred Hutch) in the national epicenter of this pandemic. Here, we summarize the experience gained during our rapid response to the COVID-19 pandemic. Our efforts were aimed at safely performing urgent and potentially life-saving stem cell transplants in the setting of pandemic-related stresses on healthcare resources and shelter-in-place public health measures. We describe the unique circumstances and challenges encountered, the current state of the program amidst evolving COVID-19 cases in our community, and the guiding principles for recovery. We also estimate the collateral impact of directing clinical resources toward COVID-19-related care on cancer patients in need of stem cell transplantation. Although our experience was influenced by specific regional and institutional factors, it may help inform how transplant programs respond to COVID-19 and future pandemics.
Conflict of interest statement
SAP receives research support from Global Life Technologies, Inc., and participates in research trials with Chimerix, Inc and Merck & Co. He also currently participates in a clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (U01-AI132004); vaccines for this trial are provided by Sanofi-Aventis. All other coauthors have no relevant competing interests to disclose.
Figures
Similar articles
-
Graft Cryopreservation Does Not Impact Overall Survival after Allogeneic Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis.Biol Blood Marrow Transplant. 2020 Jul;26(7):1312-1317. doi: 10.1016/j.bbmt.2020.04.001. Epub 2020 Apr 10. Biol Blood Marrow Transplant. 2020. PMID: 32283185 Free PMC article.
-
Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal.J Natl Compr Canc Netw. 2020 Mar 20:1-4. doi: 10.6004/jnccn.2020.7560. Online ahead of print. J Natl Compr Canc Netw. 2020. PMID: 32197238
-
Impact of Covid 19 pandemic on hematopoietic stem cell transplantation activities: Report from a single center.Transfus Apher Sci. 2023 Aug;62(4):103708. doi: 10.1016/j.transci.2023.103708. Epub 2023 Mar 29. Transfus Apher Sci. 2023. PMID: 37003931 Free PMC article.
-
Meeting the Demand for Unrelated Donors in the Midst of the COVID-19 Pandemic: Rapid Adaptations by the National Marrow Donor Program and Its Network Partners Ensured a Safe Supply of Donor Products.Transplant Cell Ther. 2021 Feb;27(2):133-141. doi: 10.1016/j.jtct.2020.10.014. Epub 2020 Dec 11. Transplant Cell Ther. 2021. PMID: 33830022 Free PMC article. Review.
-
Haploidentical stem cell transplantation-bone marrow vs peripheral blood.Transfus Apher Sci. 2018 Apr;57(2):168-170. doi: 10.1016/j.transci.2018.04.015. Epub 2018 Apr 19. Transfus Apher Sci. 2018. PMID: 29764770 Review.
Cited by
-
Patient characteristics associated with conversion from negative to positive severe acute respiratory syndrome coronavirus-2 polymerase chain reaction test results: Implications for clinical false-negativity from a single-center: A case-control study.J Med Virol. 2022 Oct;94(10):4792-4802. doi: 10.1002/jmv.27932. Epub 2022 Jun 24. J Med Virol. 2022. PMID: 35698816 Free PMC article.
-
Coronavirus disease 2019 pandemic and allogeneic hematopoietic stem cell transplantation: a single center reappraisal.Cytotherapy. 2021 Jul;23(7):635-640. doi: 10.1016/j.jcyt.2020.12.001. Epub 2020 Dec 11. Cytotherapy. 2021. PMID: 33423867 Free PMC article.
-
[Impact of SARS-CoV-2 infection on graft composition and early transplant outcomes following allogeneic hematopoietic stem cell transplantation].Zhonghua Xue Ye Xue Za Zhi. 2023 Nov 14;44(11):890-899. doi: 10.3760/cma.j.issn.0253-2727.2023.11.002. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 38185517 Free PMC article. Chinese.
-
Secondary Impact of the Coronavirus Disease 19 Pandemic on Patients and the Cellular Therapy Healthcare Ecosystem.Transplant Cell Ther. 2022 Nov;28(11):737-746. doi: 10.1016/j.jtct.2022.07.020. Epub 2022 Jul 25. Transplant Cell Ther. 2022. PMID: 35902050 Free PMC article. Review.
References
-
- Nieto Y, Patton N, Hawkins T, Spearing R, Bearman SI, Jones RB, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12:217–25. doi: 10.1016/j.bbmt.2005.10.012. - DOI - PubMed
-
- Moore KA, Lipsitch M, Barry JM, Osterholm, MT. COVID-19: the CIDRAP viewpoint. Part 1: the future of the COVID-19 pandemic: lessons learned from pandemic influenza. University of Minnesota: Minneapolis, Minnesota. Retrieved from https://www.cidrap.umn.edu/sites/default/files/public/downloads/cidrap-c.... Accessed Date: 8 Jul 2020.
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–93. - PubMed
-
- Ueda M, Martins R, Hendrie PC, McDonnell T, Crews JR, Wong TL, et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J Natl Compr Cancer Netw. 2020;18:366–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

