Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues
- PMID: 32981112
- PMCID: PMC7537546
- DOI: 10.1111/his.14264
Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues
Abstract
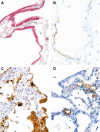
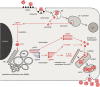
Transmission electron microscopy has become a valuable tool to investigate tissues of COVID-19 patients because it allows visualisation of SARS-CoV-2, but the 'virus-like particles' described in several organs have been highly contested. Because most electron microscopists in pathology are not accustomed to analysing viral particles and subcellular structures, our review aims to discuss the ultrastructural changes associated with SARS-CoV-2 infection and COVID-19 with respect to pathology, virology and electron microscopy. Using micrographs from infected cell cultures and autopsy tissues, we show how coronavirus replication affects ultrastructure and put the morphological findings in the context of viral replication, which induces extensive remodelling of the intracellular membrane systems. Virions assemble by budding into the endoplasmic reticulum-Golgi intermediate complex and are characterised by electron-dense dots of cross-sections of the nucleocapsid inside the viral particles. Physiological mimickers such as multivesicular bodies or coated vesicles serve as perfect decoys. Compared to other in-situ techniques, transmission electron microscopy is the only method to visualise assembled virions in tissues, and will be required to prove SARS-CoV-2 replication outside the respiratory tract. In practice, documenting in tissues the characteristic features seen in infected cell cultures seems to be much more difficult than anticipated. In our view, the hunt for coronavirus by transmission electron microscopy is still on.
Keywords: coronavirus; electron microscopy; ultrastructure; virus replication.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Battegay M, Kuehl R, Tschudin‐Sutter S et al. 2019‐novel Coronavirus (2019‐nCoV): estimating the case fatality rate – a word of caution. Swiss Med. Wkly 2020; 150; w20203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

