Inhibitor of DNA binding 2 (Id2) Regulates Photic Entrainment Responses in Mice: Differential Responses of the Id2-/- Mouse Circadian System Are Dependent on Circadian Phase and on Duration and Intensity of Light
- PMID: 32981454
- PMCID: PMC9536081
- DOI: 10.1177/0748730420957504
Inhibitor of DNA binding 2 (Id2) Regulates Photic Entrainment Responses in Mice: Differential Responses of the Id2-/- Mouse Circadian System Are Dependent on Circadian Phase and on Duration and Intensity of Light
Abstract
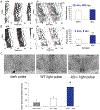
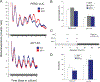
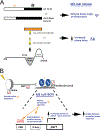
ID2 is a rhythmically expressed helix-loop-helix transcriptional repressor, and its deletion results in abnormal properties of photoentrainment. By examining parametric and nonparametric models of entrainment, we have started to explore the mechanism underlying this circadian phenotype. Id2-/- mice were exposed to differing photoperiods, and the phase angle of entrainment under short days was delayed 2 h as compared with controls. When exposed to long durations of continuous light, enhanced entrainment responses were observed after a delay of the clock but not with phase advances. However, the magnitude of phase shifts was not different in Id2-/- mice tested in constant darkness using a discrete pulse of saturating light. No differences were observed in the speed of clock resetting when challenged by a series of discrete pulses interspaced by varying time intervals. A photic phase-response curve was constructed, although no genotypic differences were observed. Although phase shifts produced by discrete saturating light pulses at CT16 were similar, treatment with a subsaturating pulse revealed a ~2-fold increase in the magnitude of the Id2-/- shift. A corresponding elevation of light-induced per1 expression was observed in the Id2-/- suprachiasmatic nucleus (SCN). To test whether the phenotype is based on a sensitivity change at the level of the retina, pupil constriction responses were measured. No differences were observed in responses or in retinal histology, suggesting that the phenotype occurs downstream of the retina and retinal hypothalamic tract. To test whether the phenotype is due to a reduced amplitude of state variables of the clock, the expression of clock genes per1 and per2 was assessed in vivo and in SCN tissue explants. Amplitude, phase, and period length were normal in Id2-/- mice. These findings suggest that ID2 contributes to a photoregulatory mechanism at the level of the SCN central pacemaker through control of the photic induction of negative elements of the clock.
Keywords: circadian rhythm; clock genes; light intensity; luciferase reporter; period gene; phase angle; phase shift; phase-response curve; photoentrainment; pupillometry.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock.Int J Mol Sci. 2021 Sep 6;22(17):9632. doi: 10.3390/ijms22179632. Int J Mol Sci. 2021. PMID: 34502541 Free PMC article.
-
A role for Id2 in regulating photic entrainment of the mammalian circadian system.Curr Biol. 2009 Feb 24;19(4):297-304. doi: 10.1016/j.cub.2008.12.052. Epub 2009 Feb 12. Curr Biol. 2009. PMID: 19217292 Free PMC article.
-
Helix-loop-helix protein Id2 stabilizes mammalian circadian oscillation under constant light conditions.Zoolog Sci. 2013 Dec;30(12):1011-8. doi: 10.2108/zsj.30.1011. Zoolog Sci. 2013. PMID: 24320178
-
[Mechanisms of structural plasticity associated with photic synchronization of the circadian clock within the suprachiasmatic nucleus].J Soc Biol. 2009;203(1):49-63. doi: 10.1051/jbio:2009004. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358811 Review. French.
-
In vivo genome-wide binding of Id2 to E2F4 target genes as part of a reversible program in mice liver.Cell Mol Life Sci. 2014 Sep;71(18):3583-97. doi: 10.1007/s00018-014-1588-1. Epub 2014 Feb 28. Cell Mol Life Sci. 2014. PMID: 24573694 Free PMC article. Review.
Cited by
-
Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock.Int J Mol Sci. 2021 Sep 6;22(17):9632. doi: 10.3390/ijms22179632. Int J Mol Sci. 2021. PMID: 34502541 Free PMC article.
-
Photic Entrainment of the Circadian System.Int J Mol Sci. 2022 Jan 10;23(2):729. doi: 10.3390/ijms23020729. Int J Mol Sci. 2022. PMID: 35054913 Free PMC article. Review.
References
-
- Adachi AA, Fujioka A, Nagano M, Masumoto KH, Takumi T, Yoshimura T, Ebihara S, Mori K, Yokota Y, and Shigeyoshi Y (2013) Helix-loop-helix protein Id2 stabilizes mammalian circadian oscillation under constant light conditions. Zoolog Sci 30:1011–1018. - PubMed
-
- Albrecht U, Sun ZS, Eichele G, and Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91:1055–1064. - PubMed
-
- Aschoff J (1979) Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 49:225–249. - PubMed
-
- Berson DM (2003) Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci 26:314–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

