Effects of PCSK9 Inhibitor on Favorable Limb Outcomes in Patients with Chronic Limb-Threatening Ischemia
- PMID: 32981918
- PMCID: PMC8265925
- DOI: 10.5551/jat.57653
Effects of PCSK9 Inhibitor on Favorable Limb Outcomes in Patients with Chronic Limb-Threatening Ischemia
Abstract
Aim: The aim of this study was to examine the effects of evolocumab on favorable limb events in patients with chronic limb-threatening ischemia (CLTI).
Methods: A single-center, prospective observational study was performed on 30 patients with CLTI. The subjects were divided into 2 groups based on evolocumab administration: evolocumab-treated (E) group ( n=14) and evolocumab non-treated (non-E) group (n=16). The primary outcome was 12-month freedom from major amputation. The secondary outcomes were 12-month amputation-free survival (AFS), overall survival (OS), and wound-free limb salvage. The mean follow-up period was 18±11 months.
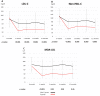
Results: No significant difference was detected between the two groups for the 12-month freedom from major amputation (log-rank p=0.15), while the 12-month AFS rate was significantly higher in the E group than that in the non-E group (log-rank p=0.02). The 12-month OS rate in the E group was shown a tendency for improvement, as compared with that in the non-E group (log-rank p=0.056). Evolocumab administration was not associated with a significant change in freedom from major amputation (HR, 0.23, 95% CI, 0.03-2.07, p=0.19). However, evolocumab administration was related to a tendency for improvement of AFS and OS (HR, 0.13, 95% CI, 0.02-1.06, p=0.056; HR, 0.16, 95% CI, 0.02-1.37, p=0.09, respectively). Moreover, The E group had a higher proportion of wound-free limb salvage at 12 months (92% vs. 42%, p=0.03).
Conclusion: Evolocumab administration was associated with a better AFS outcome in patients with CLTI. Long-term administration of evolocumab over 12 months contributed to improving proportion of wound-free limb salvage.
Keywords: Amputation; Chronic limb-threatening ischemia; Evolocumab; Peripheral artery disease; Proprotein convertase subtilisin/kexin type 9 inhibitor.
Figures


Similar articles
-
Prognostic Significance of Preoperative Functional Independence Measure (FIM) on Long-Term Outcomes in Patients with Chronic Limb-Threatening Ischemia (CLTI).Ann Vasc Surg. 2022 Jul;83:275-283. doi: 10.1016/j.avsg.2021.10.064. Epub 2021 Dec 10. Ann Vasc Surg. 2022. PMID: 34902471
-
The utility of geriatric nutritional risk index to predict outcomes in chronic limb-threatening ischemia.Catheter Cardiovasc Interv. 2022 Jan 1;99(1):121-133. doi: 10.1002/ccd.29949. Epub 2021 Sep 19. Catheter Cardiovasc Interv. 2022. PMID: 34541783
-
Comparison of Long Term Outcomes After Endovascular Treatment Versus Bypass Surgery in Chronic Limb Threatening Ischaemia Patients with Long Femoropopliteal Lesions.Eur J Vasc Endovasc Surg. 2021 Feb;61(2):258-269. doi: 10.1016/j.ejvs.2020.11.009. Epub 2020 Dec 15. Eur J Vasc Endovasc Surg. 2021. PMID: 33334672
-
Outcomes of Conservative Treatment in Patients with Chronic Limb Threatening Ischaemia: A Systematic Review and Meta-Analysis.Eur J Vasc Endovasc Surg. 2021 Aug;62(2):214-224. doi: 10.1016/j.ejvs.2021.01.005. Epub 2021 Mar 3. Eur J Vasc Endovasc Surg. 2021. PMID: 33674157
-
A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia.Eur J Vasc Endovasc Surg. 2019 Jul;58(1S):S110-S119. doi: 10.1016/j.ejvs.2019.04.013. Epub 2019 Jun 17. Eur J Vasc Endovasc Surg. 2019. PMID: 31221539
Cited by
-
Exploring research trends and hotspots on PCSK9 inhibitor studies: a bibliometric and visual analysis spanning 2007 to 2023.Front Cardiovasc Med. 2024 Nov 22;11:1474472. doi: 10.3389/fcvm.2024.1474472. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39650150 Free PMC article.
-
Long-Term Adverse Limb Events After Femoral Artery Endovascular Revascularization: The Boston FAROUT Study.J Soc Cardiovasc Angiogr Interv. 2024 Oct 2;3(10):102241. doi: 10.1016/j.jscai.2024.102241. eCollection 2024 Oct. J Soc Cardiovasc Angiogr Interv. 2024. PMID: 39525994 Free PMC article.
-
Clinical Impact of Measures for Frailty Severity in Poor-Risk Patients Undergoing Revascularization for Chronic Limb-Threatening Ischemia.J Atheroscler Thromb. 2022 Feb 1;29(2):221-228. doi: 10.5551/jat.61481. Epub 2021 Jan 30. J Atheroscler Thromb. 2022. PMID: 33518553 Free PMC article.
-
Real-World Analyses of the Treatment Conditions in Patients Initiating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor in Taiwan.J Atheroscler Thromb. 2023 Sep 1;30(9):1123-1131. doi: 10.5551/jat.63789. Epub 2022 Nov 24. J Atheroscler Thromb. 2023. PMID: 36418110 Free PMC article.
References
-
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR and Rudan I: Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. The Lancet Global Health, 2019; 7: e1020-e1030 - PubMed
-
- Criqui MH and Aboyans V: Epidemiology of peripheral artery disease. Circ Res, 2015; 116: 1509-1526 - PubMed
-
- Kithcart AP and Beckman JA: ACC/AHA Versus ESC Guidelines for Diagnosis and Management of Peripheral Artery Disease: JACC Guideline Comparison. J Am Coll Cardiol, 2018; 72: 2789-2801 - PubMed
-
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, Aboyans V, Aksoy M, Alexandrescu VA, Armstrong D, Azuma N, Belch J, Bergoeing M, Bjorck M, Chakfe N, Cheng S, Dawson J, Debus ES, Dueck A, Duval S, Eckstein HH, Ferraresi R, Gambhir R, Garguilo M, Geraghty P, Goode S, Gray B, Guo W, Gupta PC, Hinchliffe R, Jetty P, Komori K, Lavery L, Liang W, Lookstein R, Menard M, Misra S, Miyata T, Moneta G, Munoa Prado JA, Munoz A, Paolini JE, Patel M, Pomposelli F, Powell R, Robless P, Rogers L, Schanzer A, Schneider P, Taylor S, De Ceniga MV, Veller M, Vermassen F, Wang J, Wang S, Gvg Writing Group for the Joint Guidelines of the Society for Vascular Surgery ESfVS and World Federation of Vascular S: Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg, 2019; 58: S1-S109 e133 - PMC - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS and Pedersen TR: Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med, 2017; 376: 1713-1722 - PubMed

