Impact of newborn screening and quality of therapy on the neurological outcome in glutaric aciduria type 1: a meta-analysis
- PMID: 32981931
- PMCID: PMC7790745
- DOI: 10.1038/s41436-020-00971-4
Impact of newborn screening and quality of therapy on the neurological outcome in glutaric aciduria type 1: a meta-analysis
Abstract
Purpose: Glutaric aciduria type 1 (GA1), a rare inherited neurometabolic disorder, results in a complex movement disorder (MD) with predominant dystonia if untreated. Implementation into newborn screening (NBS) programs and adherence to recommended therapy are thought to improve the neurological outcome.
Methods: Systematic literature search for articles published from 2000 to 2019 was performed using the PRISMA protocol. Studies reporting on more than one individual identified by NBS were included. We investigated effects of interventional and noninterventional variables on neurological outcome.
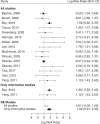
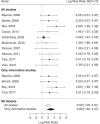
Results: Fifteen publications reporting on 647 GA1 patients were included. In the NBS group (n = 261 patients), 195 patients remained asymptomatic (74.7%), while 66 patients (25.3%) developed a MD. Compared with the NBS group, a much higher proportion of patients (349/386; 90.4%; p < 0.0001) diagnosed after the manifestation of neurologic symptoms had a MD and an abnormal motor development (285/349; 81.7%; p < 0.0001). For NBS patients, deviations from the recommended diet increased the risk of insidious onset MD, while delayed start of emergency treatment increased the risk of acute onset MD.
Conclusions: This meta-analysis demonstrates that NBS programs for GA1 have an overall positive effect on the neurological outcome of affected individuals but their success critically depends on the quality of therapy.
Keywords: glutaric acidemia type 1; glutaric aciduria type 1; newborn screening; outcome; meta-analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

