Alkaloids of Phaedranassa dubia (Kunth) J.F. Macbr. and Phaedranassa brevifolia Meerow (Amaryllidaceae) from Ecuador and its cholinesterase-inhibitory activity
- PMID: 32982003
- PMCID: PMC7500283
- DOI: 10.1016/j.sajb.2020.09.007
Alkaloids of Phaedranassa dubia (Kunth) J.F. Macbr. and Phaedranassa brevifolia Meerow (Amaryllidaceae) from Ecuador and its cholinesterase-inhibitory activity
Abstract
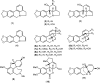
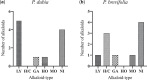
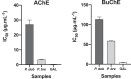
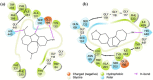
Alzheimer's disease is considered the most common cause of dementia and, in an increasingly aging population worldwide, the quest for treatment is a priority. Amaryllidaceae alkaloids are of main interest because of their cholinesterase inhibition potential, which is the main palliative treatment available for this disease. We evaluated the alkaloidal profile and the in vitro inhibitory activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) of bulb alkaloid extract of Phaedranassa dubia and Phaedranassa brevifolia collected in Ecuador. Using gas chromatography coupled to mass spectrometry (GC-MS), we identified typical Amaryllidaceae alkaloids in these species, highlighting the presence of lycorine-type alkaloids in P. dubia and haemanthamine/crinine-type in P. brevifolia. The species P. dubia and P. brevifolia showed inhibitory activities against AChE (IC50 values of 25.48 ± 0.39 and 3.45 ± 0.29 μg.mL-1, respectively) and BuChE (IC50 values of 114.96 ± 4.94 and 58.89 ± 0.55 μg.mL-1, respectively). Computational experiments allowed us to understand the interactions of the alkaloids identified in these samples toward the active sites of AChE and BuChE. In silico, some alkaloids detected in these Amaryllidaceae species presented higher estimated binding free energy toward BuChE than galanthamine. This is the first study about the alkaloid profile and biological potential of P. brevifolia species.
Keywords: AChE; AChE, Acetylcholinesterase; AE, alkaloid extract; ATCI, acetylthiocholine iodide; Alkaloids; Alzheimer's disease; Amaryllidaceae; BTCI, butyrylthiocholine iodide; BuChE; BuChE, butyrylcholinesterase; CD, circular dichroism; DTNB, (5,5′-dithio-bis-[2-nitrobenzoic acid]); Et2O, diethyl ether; EtOAc, ethyl acetate; GAL, galanthamine; GC-MS, gas chromatography coupled to mass spectrometry; IUCN, International Union for Conservation of Nature; MS, mass spectrometry; MeOH, methanol; Molecular docking; NMR, nuclear magnetic resonance; Phaedranassa; UV, ultraviolet.
© 2020 SAAB. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures


References
-
- Acosta K., Pigni N., Oleas N., Bastida J. Identification of the alkaloids of Stenomesson aurantiacum (Kunth) Herb., an Amaryllidaceae species from the Ecuadorian Andes. PharmacologyOnline. 2014;3:178–183.
-
- Adamo C., Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999;110:6158–6170.
-
- Bastida J., Berkov S., Torras L., Pigni N.B., de Andrade J.P., Martínez V., Codina C., Viladomat F. In: Recent Advances in Pharmaceutical Sciences, Transworld Research Network. Muñoz-Torrero D, editor. 2011. Chemical and biological aspects of Amaryllidaceae alkaloids; pp. 65–100. Kerala.
-
- Berkov S., Georgieva L., Kondakova V., Atanassov A., Viladomat F., Bastida J., Codina C. Plant sources of galanthamine: phytochemical and biotechnological aspects. Biotechnol. Biotechnol. Equ. 2009;23:1170–1176.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
