Golgi protein-73: A biomarker for assessing cirrhosis and prognosis of liver disease patients
- PMID: 32982114
- PMCID: PMC7495033
- DOI: 10.3748/wjg.v26.i34.5130
Golgi protein-73: A biomarker for assessing cirrhosis and prognosis of liver disease patients
Abstract
Background: Reliable biomarkers of cirrhosis, hepatocellular carcinoma (HCC), or progression of chronic liver diseases are missing. In this context, Golgi protein-73 (GP73) also called Golgi phosphoprotein-2, was originally defined as a resident Golgi type II transmembrane protein expressed in epithelial cells. As a result, GP73 expression was found primarily in biliary epithelial cells, with only slight detection in hepatocytes. However, in patients with acute or chronic liver diseases and especially in HCC, the expression of GP73 is significantly up-regulated in hepatocytes. So far, few studies have assessed GP73 as a diagnostic or prognostic marker of liver fibrosis and disease progression.
Aim: To assess serum GP73 efficacy as a diagnostic marker of cirrhosis and/or HCC or as predictor of liver disease progression.
Methods: GP73 serum levels were retrospectively determined by a novel GP73 ELISA (QUANTA Lite® GP73, Inova Diagnostics, Inc., Research Use Only) in a large cohort of 632 consecutive patients with chronic viral and non-viral liver diseases collected from two tertiary Academic centers in Larissa, Greece (n = 366) and Debrecen, Hungary (n = 266). Aspartate aminotransferase (AST)/Platelets (PLT) ratio index (APRI) was also calculated at the relevant time points in all patients. Two hundred and three patients had chronic hepatitis B, 183 chronic hepatitis C, 198 alcoholic liver disease, 28 autoimmune cholestatic liver diseases, 15 autoimmune hepatitis, and 5 with other liver-related disorders. The duration of follow-up was 50 (57) mo [median (interquartile range)]. The development of cirrhosis, liver decompensation and/or HCC during follow-up were assessed according to internationally accepted guidelines. In particular, the surveillance for the development of HCC was performed regularly with ultrasound imaging and alpha-fetoprotein (AFP) determination every 6 mo in cirrhotic and every 12 mo in non-cirrhotic patients.
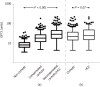
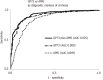
Results: Increased serum levels of GP73 (> 20 units) were detected at initial evaluation in 277 out of 632 patients (43.8%). GP73-seropositivity correlated at baseline with the presence of cirrhosis (96.4% vs 51.5%, P < 0.001), decompensation of cirrhosis (60.3% vs 35.5%, P < 0.001), presence of HCC (18.4% vs 7.9%, P < 0.001) and advanced HCC stage (52.9% vs 14.8%, P = 0.002). GP73 had higher diagnostic accuracy for the presence of cirrhosis compared to APRI score [Area under the curve (AUC) (95%CI): 0.909 (0.885-0.934) vs 0.849 (0.813-0.886), P = 0.003]. Combination of GP73 with APRI improved further the accuracy (AUC: 0.925) compared to GP73 (AUC: 0.909, P = 0.005) or APRI alone (AUC: 0.849, P < 0.001). GP73 levels were significantly higher in HCC patients compared to non-HCC [22.5 (29.2) vs 16 (20.3) units, P < 0.001) and positively associated with BCLC stage [stage 0: 13.9 (10.8); stage A: 17.1 (16.8); stage B: 19.6 (22.3); stage C: 32.2 (30.8); stage D: 45.3 (86.6) units, P < 0.001] and tumor dimensions [very early: 13.9 (10.8); intermediate: 19.6 (18.4); advanced: 29.1 (33.6) units, P = 0.004]. However, the discriminative ability for HCC diagnosis was relatively low [AUC (95%CI): 0.623 (0.570-0.675)]. Kaplan-Meier analysis showed that the detection of GP73 in patients with compensated cirrhosis at baseline, was prognostic of higher rates of decompensation (P = 0.036), HCC development (P = 0.08), and liver-related deaths (P < 0.001) during follow-up.
Conclusion: GP73 alone appears efficient for detecting cirrhosis and superior to APRI determination. In combination with APRI, its diagnostic performance can be further improved. Most importantly, the simple GP73 measurement proved promising for predicting a worse outcome of patients with both viral and non-viral chronic liver diseases.
Keywords: Aspartate aminotransferase/Platelets ratio index score; Biomarker; Cirrhosis; Golgi protein-73; Hepatic fibrosis; Hepatitis B; Hepatitis C; Hepatocellular carcinoma.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not -for-profit sectors. However, Inova Diagnostics Inc. provided funds to Norman GL, Shums Z and Albesa R who are employees of Inova (ELISA kits), for the support of this study. All other authors have no disclosures relevant to this manuscript. Other authors have declared that no competing interest exists.
Figures


References
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Zachou K, Gabeta S, Shums Z, Gatselis NK, Koukoulis GK, Norman GL, Dalekos GN. COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur J Intern Med. 2017;38:83–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

