Catalytic Enantioselective Synthesis of Chiral Organofluorine Compounds: Alcohol-Mediated Hydrogen Transfer for Catalytic Carbonyl Reductive Coupling
- PMID: 32982140
- PMCID: PMC7516892
- DOI: 10.1021/acs.oprd.9b00035
Catalytic Enantioselective Synthesis of Chiral Organofluorine Compounds: Alcohol-Mediated Hydrogen Transfer for Catalytic Carbonyl Reductive Coupling
Abstract
Alcohol-mediated carbonyl addition has enabled catalytic enantioselective syntheses of diverse fluorine-containing compounds without the need for stoichiometric metals or discrete redox manipulations. Reactions of this type may be separated into two broad categories: redox-neutral hydrogen auto-transfer reactions wherein lower alcohols and n-unsaturated pronucleophiles are converted to higher alcohols and corresponding 2-propanol mediated carbonyl reductive couplings.
Keywords: carbonyl addition; enantioselective catalysis; fluorine; hydrogen transfer; iridium; ruthenium.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


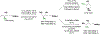


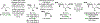
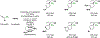

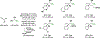


References
-
- For selected reviews underscoring he prevalence of organofluorine compounds in pharmaceutical and agrochemical ingredients:
- Thayer AM ENZYMES AT WORK: Rapid screening and optimization of enzymatic activity, along with available, easy-to-use enzymes, are making biocatalysis a handy tool for chiral synthesis. Chem. Eng. News 2006, 84, 15–25.
- Müller K; Faeh C; Diederich F Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. - PubMed
- Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. - PubMed
- Zhou Y; Wang J; Gu Z; Wang S; Zhu W; Aceña JL; Soloshonok VA; Izawa K; Liu H Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. - PubMed
- Meanwell NA Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. - PubMed
-
- For selected reviews on the preparation of organofluorine compounds, see:
- Gong Y Recent Applications of Trifluoroacetaldehyde Ethyl Hemiacetal for the Synthesis of Trifluoromethylated Compounds. Curr. Org. Chem. 2004, 17, 1659–1675.
- Cahard D; Xu X; Couve-Bonnaire S; Pannecoucke X Fluorine & chirality: how to create a nonracemic stereogenic carbon–fluorine centre? Chem. Soc. Rev. 2010, 39, 558–568. - PubMed
- Nie J; Guo H-C; Cahard D; Ma J-A Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates. Chem. Rev. 2011, 111, 455–529. - PubMed
- Furuya T; Kamlet AS; Ritter T Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. - PMC - PubMed
- Besset T; Schneider C; Cahard D Tamed arene and heteroarene trifluoromethylation. Angew. Chem. Int. Ed. 2012, 51, 5048–5050. - PubMed
- Studer AA “Renaissance” in radical trifluoromethylation. Angew. Chem. Int. Ed. 2012, 51, 8950–8958. - PubMed
- Liang T; Neumann CN; Ritter T Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. - PubMed
- Campbell MG; Ritter T Modern Carbon–Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis. Chem. Rev. 2015, 115, 612–633. - PubMed
- Xu X-H; Matsuzaki K; Shibata N Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions . Chem. Rev. 2015, 115, 731–764. - PubMed
- Yang X; Wu T; Phipps RJ; Toste FD Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870. - PMC - PubMed
- Alonso C; Martinez de Marigorta E; Rubiales G; Palacios F Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. - PubMed
- Zhu Y; Han J; Wang J; Shibata N; Sodeoka M; Soloshonok VA; Coelho JAS; Toste FD Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev. 2018, 118, 3887–3964. - PMC - PubMed
-
- For selected reviews on alcohol-mediated carbonyl addition, see:
- Hassan A; Krische MJ Unlocking Hydrogenation for C–C Bond Formation: A Brief Overview of Enantioselective Methods. Org. Proc. Res. Devel. 2011, 15, 1236–1242. - PMC - PubMed
- Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C–H Functionalization of Alcohols by Redox‐Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem. Int. Ed. 2014, 53, 9142–9150. - PMC - PubMed
- Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354, aah5133–1–aah5133–5. - PMC - PubMed
- Kim SW; Zhang W; Krische MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res. 2017, 50, 2371–2380. - PMC - PubMed
- Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052. - PMC - PubMed
-
- For selected reviews on alcohol substitution via “borrowing hydrogen” or hydrogen auto-transfer, see:
- Guillena G; Ramón DJ; Yus M Alcohols as Electrophiles in C–C Bond Forming Reactions: The Hydrogen Autotransfer Process. Angew. Chem. Int. Ed. 2007, 46, 2358–2364. - PubMed
- Hamid MHSA; Slatford PA; Williams JMJ Borrowing Hydrogen in the Activation of Alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575.
- Nixon TD; Whittlesey MK; Williams JMJ Transition Metal Catalyzed Reactions of Alcohols using Borrowing Hydrogen Methodology. Dalton Trans. 2009, 753–762. - PubMed
- Dobereiner GE; Crabtree RH Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. - PubMed
- Guillena G; Ramón DJ; Yus M Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Alcohols and Amines as Electrophiles. Chem. Rev. 2010, 110, 1611–1641. - PubMed
- Yang Q; Wang Q; Yu Z Substitution of Alcohols by N-Nucleophiles via Transition Metal-Catalyzed Dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. - PubMed
- Nandakumar A; Midya SP; Landge VG; Balaraman E Transition-Metal-Catalyzed Hydrogen-Transfer Annulations: Access to Heterocyclic Scaffolds. Angew. Chem. Int. Ed. 2015, 54, 11022–11034. - PubMed
- Huang F; Liu Z; Yu Z C-Alkylation of Ketones and Related Compounds by Alcohols: Transition-Metal-Catalyzed Dehydrogenation. Angew. Chem. Int. Ed. 2016, 55, 862–875. - PubMed
- Quintard A; Rodriguez J Catalytic Enantioselective OFF ↔ ON Activation Processes Initiated by Hydrogen Transfer: Concepts and Challenges. Chem. Comm. 2016, 52, 10456–10473. - PubMed
- Quintard A; Rodriguez J A Step into an Eco-Compatible Future: Iron- and Cobalt-Catalyzed Borrowing Hydrogen Transformation. ChemSusChem 2016, 9, 28–30. - PubMed
- Chelucci G Metal-Catalyzed Dehydrogenative Synthesis of Pyrroles and Indoles from Alcohols. Coord. Chem. Rev. 2017, 331, 37–53.
-
- Kim IS; Ngai M-Y; Krische MJ Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Using Allyl Acetate as an Allyl Metal Surrogate. J. Am. Chem. Soc. 2008, 130, 6340–6341. - PMC - PubMed
- Kim IS; Ngai M-Y; Krische MJ Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Allyl Acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition. J. Am. Chem. Soc. 2008, 130, 14891–14899. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
