Human Secretary Phospholipase A2 Mutations and Their Clinical Implications
- PMID: 32982370
- PMCID: PMC7502393
- DOI: 10.2147/JIR.S269557
Human Secretary Phospholipase A2 Mutations and Their Clinical Implications
Abstract
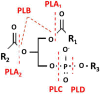
Phospholipases A2 (PLA2s) belong to a superfamily of enzymes responsible for hydrolysis of the sn-2 fatty acids of membrane phospholipids to release arachidonic acid. PLA2s are the rate limiting enzyme for the downstream synthesis of prostaglandins and leukotrienes that are the main mediators of inflammation. The extracellular forms of this enzyme are also called the secretary phospholipase A2 (sPLA2) and are distributed extensively in most of the tissues in the human body. Their integral role in inflammatory pathways has been the primary reason for the extensive research on this molecule. The catalytic mechanism of sPLA2 is initiated by a histidine/aspartic acid/calcium complex within the active site. Though they are known to have certain housekeeping functions, certain mutations of sPLA2 are known to be implicated in causation of certain pathologies leading to diseases such as atherosclerosis, cardiovascular diseases, benign fleck retina, neurodegeneration, and asthma. We present an overview of human sPLA2 and a comprehensive compilation of the mutations that result in various disease phenotypes. The study not only helps to have a holistic understanding of human sPLA2 mutations and their clinical implications, but is also a useful platform to initiate research pertaining to structure-function relationship of the mutations to develop effective therapies for management of these diseases.
Keywords: clinical implications; mutations; sPLA2; secretary phospholipase A2; structure–function relationship.
© 2020 Khan and Hariprasad.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Van Deenen L, Haas G. The substrate specificity of phospholipase A. Biochimica Et Biophysica Acta (BBA) Spec Section Lipids Rel Sub. 1963;70:538–553. - PubMed
-
- Wittcoff H. The phosphatides. The Phosphatides. 1951.
-
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269(18):13057–13060. - PubMed
-
- Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. - PubMed
-
- Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica Et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2006;1761(11):1246–1259. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

