Microtransesophageal Echocardiographic Guidance during Percutaneous Interatrial Septal Closure without General Anaesthesia
- PMID: 32982607
- PMCID: PMC7492935
- DOI: 10.1155/2020/1462140
Microtransesophageal Echocardiographic Guidance during Percutaneous Interatrial Septal Closure without General Anaesthesia
Abstract
Objective: To study the safety and efficacy of microtransesophageal echocardiography (micro-TEE) and TEE during percutaneous atrial septal defect (ASD) and patent foramen ovale (PFO) closure.
Background: TEE has proven to be safe during ASD and PFO closure under general anaesthesia. Micro-TEE makes it possible to perform these procedures under local anaesthesia. We are the first to describe the safety and efficacy of micro-TEE for percutaneous closure.
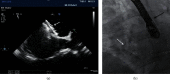
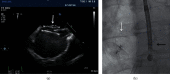
Methods: All consecutive patients who underwent ASD and PFO closure between 2013 and 2018 were included. The periprocedural complications were registered. Residual shunts were diagnosed using transthoracic contrast echocardiography (TTCE). All data were compared between the use of TEE or micro-TEE within the ASD and PFO groups separately.
Results: In total, 82 patients underwent ASD closure, 46 patients (49.1 ± 15.0 years) with TEE and 36 patients (47.8 ± 12.1 years) using micro-TEE guidance. Median device diameter was, respectively, 26 mm (range 10-40 mm) and 27 mm (range 10-35 mm). PFO closure was performed in 120 patients, 55 patients (48.6 ± 9.2 years, median device diameter 25 mm, range 23-35 mm) with TEE and 65 patients (mean age 51.0 ± 11.8 years, median device diameter 27 mm, range 23-35 mm) using micro-TEE. There were no major periprocedural complications, especially no device embolizations within all groups. Six months after closure, there was no significant difference in left-to-right shunt after ASD closure and no significant difference in right-to-left shunt after PFO closure using TEE or micro-TEE.
Conclusion: Micro-TEE guidance without general anaesthesia during percutaneous ASD and PFO closure is as safe as TEE, without a significant difference in the residual shunt rate after closure.
Copyright © 2020 Roel J. R. Snijder et al.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Alqahtani F., Bhirud A., Aljohani S., et al. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: nationwide trend and comparative analysis. Journal of Interventional Cardiology. 2017;30(3):234–241. doi: 10.1111/joic.12382. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
