Multiple Origins of Secretagogin Expressing Cortical GABAergic Neuron Precursors in the Early Human Fetal Telencephalon
- PMID: 32982702
- PMCID: PMC7492523
- DOI: 10.3389/fnana.2020.00061
Multiple Origins of Secretagogin Expressing Cortical GABAergic Neuron Precursors in the Early Human Fetal Telencephalon
Abstract
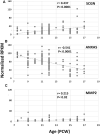
Secretagogin (SCGN) which acts as a calcium signaling sensor, has previously been shown to be expressed by a substantial population of cortical GABAergic neurons at mid-gestation in humans but not in mice. The present study traced SCGN expression in cortical GABAergic neurons in human fetal forebrain from earlier stages than previously studied. Multiple potential origins of SCGN-expressing neurons were identified in the caudal ganglionic eminence (CGE) lateral ganglionic eminence (LGE) septum and preoptic area; these cells largely co-expressed SP8 but not the medial ganglionic eminence marker LHX6. They followed various migration routes to reach their target regions in the neocortex, insular and olfactory cortex (OC) and olfactory bulbs. A robust increase in the number of SCGN-expressing GABAergic cortical neurons was observed in the midgestational period; 58% of DLX2+ neurons expressed SCGN in the cortical wall at 19 post-conceptional weeks (PCW), a higher proportion than expressed calretinin, a marker for GABAergic neurons of LGE/CGE origin. Furthermore, although most SCGN+ neurons co-expressed calretinin in the cortical plate (CP) and deeper layers, in the marginal zone (MZ) SCGN+ and calretinin+ cells formed separate populations. In the adult mouse, it has previously been shown that in the rostral migratory stream (RMS), SCGN, annexin V (ANXA5), and matrix metalloprotease 2 (MMP2) are co-expressed forming a functioning complex that exocytoses MMP2 in response to calcium. In the present study, ANXA5 showed widespread expression throughout the cortical wall, although MMP2 expression was very largely limited to the CP. We found co-expression of these proteins in some SCGN+ neurons in the subventricular zones (SVZ) suggesting a limited role for these cells in remodeling the extracellular matrix, perhaps during cell migration.
Keywords: annexin V; cerebral cortex; ganglionic eminences; inhibitory interneurons; matrix metalloprotease 2; preoptic area; secretagogin; septum.
Copyright © 2020 Alzu’bi and Clowry.
Figures




References
-
- Alzu’bi A., Lindsay S., Kerwin J., Looi S. J., Khalil F., Clowry G. J. (2017). Distinct cortical and sub-cortical neurogenic domains for GABAergic interneuron precursor transcription factors NKX2. 1, OLIG2 and COUP-TFII in early fetal human telencephalon. Brain Struct. Funct. 222, 2309–2328. 10.1007/s00429-016-1343-5 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

