Capsaicin Is a Negative Allosteric Modulator of the 5-HT3 Receptor
- PMID: 32982728
- PMCID: PMC7490547
- DOI: 10.3389/fphar.2020.01274
Capsaicin Is a Negative Allosteric Modulator of the 5-HT3 Receptor
Abstract
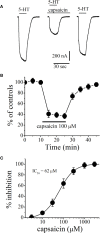
In this study, effects of capsaicin, an active ingredient of the capsicum plant, were investigated on human 5-hydroxytryptamine type 3 (5-HT3) receptors. Capsaicin reversibly inhibited serotonin (5-HT)-induced currents recorded by two-electrode voltage clamp method in Xenopus oocytes. The inhibition was time- and concentration-dependent with an IC50 = 62 μM. The effect of capsaicin was not altered in the presence of capsazepine, and by intracellular BAPTA injections or trans-membrane potential changes. In radio-ligand binding studies, capsaicin did not change the specific binding of the 5-HT3 antagonist [3H]GR65630, indicating that it is a noncompetitive inhibitor of 5-HT3 receptor. In HEK-293 cells, capsaicin inhibited 5-HT3 receptor induced aequorin luminescence with an IC50 of 54 µM and inhibition was not reversed by increasing concentrations of 5-HT. In conclusion, the results indicate that capsaicin acts as a negative allosteric modulator of human 5-HT3 receptors.
Keywords: 5-HT3 receptor; HEK-293 cells; Xenopus oocytes; allosteric modulator; capsaicin; docking; serotonin.
Copyright © 2020 Nebrisi, Prytkova, Lorke, Howarth, Alzaabi, Yang, Howarth and Oz.
Figures




References
-
- Alzaabi A. H., Howarth L., El Nebrisi E., Syed N., Susan Yang K. H., Howarth F. C., et al. (2019). Capsaicin inhibits the function of α7-nicotinic acetylcholine receptors expressed in Xenopus oocytes and rat hippocampal neurons. Eur. J. Pharmacol. 857, 172411. 10.1016/j.ejphar.2019.172411 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

