Ketamine Rescues Hippocampal Reelin Expression and Synaptic Markers in the Repeated-Corticosterone Chronic Stress Paradigm
- PMID: 32982757
- PMCID: PMC7493014
- DOI: 10.3389/fphar.2020.559627
Ketamine Rescues Hippocampal Reelin Expression and Synaptic Markers in the Repeated-Corticosterone Chronic Stress Paradigm
Abstract
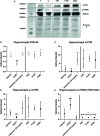
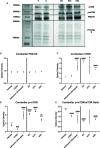
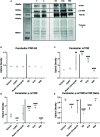
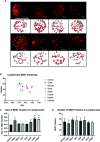
Depression is the leading cause of disability worldwide, which necessitates novel therapeutics and biomarkers to approach treatment of this neuropsychiatric disorder. To assess potential mechanisms underlying the fast-acting antidepressant actions of ketamine we used a repeated corticosterone paradigm in adult male rats to assess the effects of ketamine on reelin-positive cells, a protein largely implicated in the pathophysiology of depression. We also assessed the effects of reelin and ketamine on hippocampal and cerebellar synpatosomes, and on serotonin transporter clustering in peripheral lymphocytes to determine reelin and ketamine's impact at the synaptic and peripheral levels. Reelin and ketamine similarly rescue synaptic expression of mTOR and p-mTOR that were decreased by corticosterone. Reelin, but not ketamine, was able to rescue patterns of serotonin transporter clustering in the periphery. These findings display ketamine as a powerful modulator of reelin expression and lend strength to further evaluation of the putative fast antidepressant-like actions of reelin.
Keywords: antidepressant; hippocampus; ketamine; lymphocytes; mammalian target of rapamycin; reelin; synaptosomes.
Copyright © 2020 Johnston, Thacker, Desjardins, Kulyk, Romay-Tallon, Kalynchuk and Caruncho.
Figures


References
-
- Brymer K. J., Fenton E. Y., Kalynchuk L. E., Caruncho H. J. (2018). Peripheral Etanercept Administration Normalizes Behavior, Hippocampal Neurogenesis, and Hippocampal Reelin and GABAA Receptor Expression in a Preclinical Model of Depression. Front. Pharmacol. 9, 121. 10.3389/fphar.2018.00121 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

