The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer Within the Liver
- PMID: 32982772
- PMCID: PMC7485256
- DOI: 10.3389/fphys.2020.00990
The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer Within the Liver
Abstract
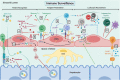
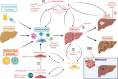
Liver sinusoidal endothelial cells (LSEC) form a unique barrier between the liver sinusoids and the underlying parenchyma, and thus play a crucial role in maintaining metabolic and immune homeostasis, as well as actively contributing to disease pathophysiology. Whilst their endocytic and scavenging function is integral for nutrient exchange and clearance of waste products, their capillarisation and dysfunction precedes fibrogenesis. Furthermore, their ability to promote immune tolerance and recruit distinct immunosuppressive leukocyte subsets can allow persistence of chronic viral infections and facilitate tumour development. In this review, we present the immunological and barrier functions of LSEC, along with their role in orchestrating fibrotic processes which precede tumourigenesis. We also summarise the role of LSEC in modulating the tumour microenvironment, and promoting development of a pre-metastatic niche, which can drive formation of secondary liver tumours. Finally, we summarise closely inter-linked disease pathways which collectively perpetuate pathogenesis, highlighting LSEC as novel targets for therapeutic intervention.
Keywords: capillarisation; endothelial dysfunction; fibrosis; hepatocellular carcinoma; inflammation; leukocyte recruitment; liver sinusoidal endothelial cell; metastasis.
Copyright © 2020 Wilkinson, Qurashi and Shetty.
Figures



References
-
- Adachi Y., Takeuchi T., Sonobe H., Ohtsuki Y. (2006). An adiponectin receptor, T-cadherin, was selectively expressed in intratumoral capillary endothelial cells in hepatocellular carcinoma: possible cross talk between T-cadherin and FGF-2 pathways. Virchows Archiv. 448 311–318. 10.1007/s00428-005-0098-9 - DOI - PubMed
-
- Alexiou D., Karayiannakis A., Syrigos K., Zbar A., Kremmyda A., Bramis I., et al. (2001). Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur. J. Cancer 37 2392–2397. 10.1016/S0959-8049(01)00318-5 - DOI - PubMed
-
- Arteta B., Lasuen N., Lopategi A., Sveinbjörnsson B., Smedsrød B., Vidal-Vanaclocha F. (2010). Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology 51 2172–2182. 10.1002/hep.23590 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

