Reappraisal of Oral Steroid Therapy for Myasthenia Gravis
- PMID: 32982912
- PMCID: PMC7477376
- DOI: 10.3389/fneur.2020.00868
Reappraisal of Oral Steroid Therapy for Myasthenia Gravis
Abstract
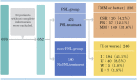
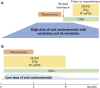
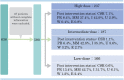
Treatment with oral corticosteroids at high doses with an escalation and de-escalation schedule is effective against myasthena gravis (MG). In fact, the use of corticosteroids has led to a reduction in mortality to below 10% after the 1960s. However, long-term use of oral steroids above a certain dosage level is known to cause a number of problems. In 2014, the Japanese clinical guidelines for MG proposed that the first goal in MG treatment (treatment target) should be set at minimal manifestations (MM) with oral prednisolone (PSL) 5 mg/day or below, and that treatment strategies should strive to attain this level as rapidly as possible. In 2015, a multicenter, cross-sectional study revealed that higher PSL dose and longer PSL treatment do not ensure better outcome. In the absence of good response, the PSL dose should be decreased by combining with modalities such as plasma exchange/plasmapheresis and intravenous immunoglobulin (fast-acting treatments). In 2018, we conducted a multicenter, cross-sectional study in a large population of Japanese patients with generalized MG, aiming to elucidate the correlation between oral PSL regimens and achievement of treatment goals. The ORs for low vs. high dose to achieve treatment goals at 1, 2, and 3 years were 10.4, 2.75, and 1.86, respectively, whereas the corresponding ORs for low vs. medium dose were 13.4, 3.99, and 4.92. Early combination with fast-acting therapy (OR 2.19 at 2 years, 2.11 at 3 years) or combination with calcineurin inhibitors (OR 2.09 at 2 years, 2.36 at 3 years) were also positively associated with achieving treatment goals. These results indicate that early combination of low-dose PSL regimens with other therapies is the key for early achievement of treatment goals in generalized MG. However, even with this regimen, ~35% of patients did not achieve the treatment target after 3 years. These results suggest the limitation of the current oral corticosteroid therapy. We need to develop new treatment options to increase the rate of satisfactory outcome.
Keywords: cross-sectional study; logistic regression analysis; myasthenia gravis; oral corticosteroids; treatment strategies.
Copyright © 2020 Imai, Suzuki, Nagane, Uzawa, Murai and Utsugisawa.
Figures
Similar articles
-
Oral corticosteroid dosing regimen and long-term prognosis in generalised myasthenia gravis: a multicentre cross-sectional study in Japan.J Neurol Neurosurg Psychiatry. 2018 May;89(5):513-517. doi: 10.1136/jnnp-2017-316625. Epub 2017 Nov 24. J Neurol Neurosurg Psychiatry. 2018. PMID: 29175893 Free PMC article.
-
Oral corticosteroid therapy and present disease status in myasthenia gravis.Muscle Nerve. 2015 May;51(5):692-6. doi: 10.1002/mus.24438. Epub 2015 Feb 4. Muscle Nerve. 2015. PMID: 25155615
-
[Correlation between oral corticosteroid therapy and present disease status in myasthenia gravis].Rinsho Shinkeigaku. 2013;53(11):1306-8. doi: 10.5692/clinicalneurol.53.1306. Rinsho Shinkeigaku. 2013. PMID: 24291969 Japanese.
-
Rationale for the clinical guidelines for myasthenia gravis in Japan.Ann N Y Acad Sci. 2018 Feb;1413(1):35-40. doi: 10.1111/nyas.13544. Epub 2018 Jan 28. Ann N Y Acad Sci. 2018. PMID: 29377151 Review.
-
[Treatment of myasthenia gravis].Rinsho Shinkeigaku. 1999 Dec;39(12):1222-5. Rinsho Shinkeigaku. 1999. PMID: 10791082 Review. Japanese.
Cited by
-
Corticosteroid Treatment-Resistance in Myasthenia Gravis.Front Neurol. 2022 Apr 25;13:886625. doi: 10.3389/fneur.2022.886625. eCollection 2022. Front Neurol. 2022. PMID: 35547366 Free PMC article. Review.
-
Myasthenia Gravis: Novel Findings and Perspectives on Traditional to Regenerative Therapeutic Interventions.Aging Dis. 2023 Aug 1;14(4):1070-1092. doi: 10.14336/AD.2022.1215. Aging Dis. 2023. PMID: 37163445 Free PMC article.
-
Impact of 2014 Japanese practice guidelines on treatment patterns in patients with myasthenia gravis: an insurance claims database study.BMJ Open. 2025 Jun 22;15(6):e095496. doi: 10.1136/bmjopen-2024-095496. BMJ Open. 2025. PMID: 40545307 Free PMC article.
-
Long-Term Improvement in a Chinese Cohort of Glucocorticoid-Resistant Childhood-Onset Myasthenia Gravis Patients Treated With Tacrolimus.Front Neurol. 2022 Feb 8;13:820205. doi: 10.3389/fneur.2022.820205. eCollection 2022. Front Neurol. 2022. PMID: 35211085 Free PMC article.
-
Adverse Side Effects Associated with Corticosteroid Therapy: A Study in 39 Patients with Generalized Myasthenia Gravis.Med Sci Monit. 2021 Oct 28;27:e933296. doi: 10.12659/MSM.933296. Med Sci Monit. 2021. PMID: 34707081 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

