Transcutaneous Auricular Vagal Nerve Stimulation and Disorders of Consciousness: A Hypothesis for Mechanisms of Action
- PMID: 32982941
- PMCID: PMC7477388
- DOI: 10.3389/fneur.2020.00933
Transcutaneous Auricular Vagal Nerve Stimulation and Disorders of Consciousness: A Hypothesis for Mechanisms of Action
Abstract
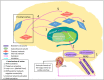
Disorders of consciousness (DoC) are the hallmark of severe acquired brain injuries characterized by abnormal activity in important brain areas and disruption within and between brain networks. As DoC's therapeutic arsenal is limited, new potential therapies such as transcutaneous auricular vagal nerve stimulation (taVNS) have recently been explored. The potential of taVNS in the process of consciousness recovery has been highlighted in recent studies with DoC patients. However, it is not clear how taVNS plays a role in the recovery of consciousness. In this article, we first describe the neural correlates of consciousness, the vagus nerve anatomy and functions, along with the results of functional magnetic resonance imaging studies using taVNS. Based on consciousness recovery and taVNS mechanisms, we propose the Vagal Cortical Pathways model. This model highlights four consecutive pathways (A. Lower brainstem activation, B. Upper brainstem activation, C. Norepinephrine pathway, and D. Serotonin pathway) likely to have an impact on patients with a brain injury and DoC. Additionally, we suggest six different mechanisms of action: (1) Activation of the ascending reticular activating system; (2) Activation of the thalamus; (3) Re-establishment of the cortico-striatal-thalamic-cortical loop; (4) Promotion of negative connectivity between external and default mode networks by the activation of the salience network; (5) Increase in activity and connectivity within the external network through the norepinephrine pathway; and (6) Increase in activity within the default mode network through the serotonin pathway. This model aims to explain the potential therapeutic effects that taVNS has on brain activity in the process of consciousness recovery.
Keywords: brain injury; brain network; disorders of consciousness; functional magnetic resonance imaging; non-invasive brain stimulation; post-coma; transcutaneous auricular vagal nerve stimulation.
Copyright © 2020 Briand, Gosseries, Staumont, Laureys and Thibaut.
Figures

References
-
- Brain Injury Association of America ABI vs. TBI: What Is the Difference? (2020). Available online at: https://www.biausa.org/brain-injury/about-brain-injury/nbiic/what-is-the... (accessed June 26, 2020).
LinkOut - more resources
Full Text Sources
Medical
Research Materials

