Exercise-Induced Improvements to Whole Body Glucose Metabolism in Type 2 Diabetes: The Essential Role of the Liver
- PMID: 32982968
- PMCID: PMC7484211
- DOI: 10.3389/fendo.2020.00567
Exercise-Induced Improvements to Whole Body Glucose Metabolism in Type 2 Diabetes: The Essential Role of the Liver
Abstract
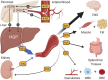
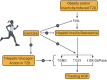
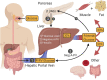
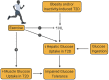
Type 2 diabetes (T2D) is a metabolic disease characterized by obesity, insulin resistance, and the dysfunction of several key glucoregulatory organs. Among these organs, impaired liver function is recognized as one of the earliest contributors to impaired whole-body glucose homeostasis, with well-characterized hepatic insulin resistance resulting in elevated rates of hepatic glucose production (HGP) and fasting hyperglycemia. One portion of this review will provide an overview of how HGP is regulated during the fasted state in healthy humans and how this process becomes dysregulated in patients with T2D. Less well-appreciated is the liver's role in post-prandial glucose metabolism, where it takes up and metabolizes one-third of orally ingested glucose. An abundance of literature has shown that the process of hepatic glucose uptake is impaired in patients with T2D, thereby contributing to glucose intolerance. A second portion of this review will outline how hepatic glucose uptake is regulated during the post-prandial state, and how it becomes dysfunctional in patients with T2D. Finally, it is well-known that exercise training has an insulin-sensitizing effect on the liver, which contributes to improved whole-body glucose metabolism in patients with T2D, thereby making it a cornerstone in the management of the disease. To this end, the impact of exercise on hepatic glucose metabolism will be thoroughly discussed, referencing key findings in the literature. At the same time, sources of heterogeneity that contribute to inconsistent findings in the field will be pointed out, as will important topics for future investigation.
Keywords: aerobic exercise; fasting blood glucose levels; glucagon; hepatic glucose production; post-prandial glucose; resistance training.
Copyright © 2020 Warner, Yao, Cason and Winnick.
Figures
Similar articles
-
Aerobic exercise training improves hepatic and muscle insulin sensitivity, but reduces splanchnic glucose uptake in obese humans with type 2 diabetes.Nutr Diabetes. 2019 Sep 2;9(1):25. doi: 10.1038/s41387-019-0090-0. Nutr Diabetes. 2019. PMID: 31474750 Free PMC article.
-
Diabetes and branched-chain amino acids: What is the link?J Diabetes. 2018 May;10(5):350-352. doi: 10.1111/1753-0407.12645. Epub 2018 Feb 13. J Diabetes. 2018. PMID: 29369529
-
Roux-en-Y gastric bypass surgery improves hepatic glucose metabolism and reduces plasma kisspeptin levels in morbidly obese patients with type 2 diabetes.Am J Physiol Gastrointest Liver Physiol. 2020 Feb 1;318(2):G370-G374. doi: 10.1152/ajpgi.00224.2019. Epub 2019 Nov 11. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31709832 Free PMC article.
-
The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes.Curr Opin Pharmacol. 2020 Dec;55:165-174. doi: 10.1016/j.coph.2020.10.016. Epub 2020 Dec 2. Curr Opin Pharmacol. 2020. PMID: 33278735 Review.
-
Is hepatic glucose production increased in type 2 diabetes mellitus?Curr Diab Rep. 2002 Jun;2(3):231-6. doi: 10.1007/s11892-002-0088-0. Curr Diab Rep. 2002. PMID: 12643178 Review.
Cited by
-
Different Effects of Leucine Supplementation and/or Exercise on Systemic Insulin Sensitivity in Mice.Front Endocrinol (Lausanne). 2021 May 12;12:651303. doi: 10.3389/fendo.2021.651303. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34054726 Free PMC article.
-
Inhibition of Hyperglycemia and Hyperlipidemia by Blocking Toll-like Receptor 4: Comparison of Wild-Type and Toll-like Receptor 4 Gene Knockout Mice on Obesity and Diabetes Modeling.Biology (Basel). 2024 Jan 22;13(1):63. doi: 10.3390/biology13010063. Biology (Basel). 2024. PMID: 38275739 Free PMC article.
-
Molecular Regulation of Skeletal Muscle Growth and Organelle Biosynthesis: Practical Recommendations for Exercise Training.Int J Mol Sci. 2021 Mar 8;22(5):2741. doi: 10.3390/ijms22052741. Int J Mol Sci. 2021. PMID: 33800501 Free PMC article. Review.
-
Advanced maternal age, overweight and obese positively correlate to the abnormal plasma glucose among gestational diabetes mellitus women even with physical exercise > 90 min/day: a prospective cohort study in Shanghai.Sci Rep. 2025 Jul 1;15(1):21191. doi: 10.1038/s41598-025-09097-6. Sci Rep. 2025. PMID: 40594962 Free PMC article.
-
[Latest Findings on the Role of α-Ketoglutarate in Metabolic Syndrome].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):783-792. doi: 10.12182/20240560302. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948289 Free PMC article. Review. Chinese.

