Antibacterial Fusion Proteins Enhance Moraxella catarrhalis Killing
- PMID: 32983170
- PMCID: PMC7492680
- DOI: 10.3389/fimmu.2020.02122
Antibacterial Fusion Proteins Enhance Moraxella catarrhalis Killing
Abstract
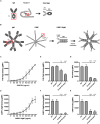
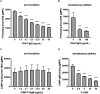
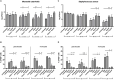
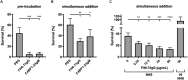
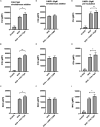
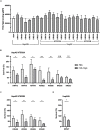
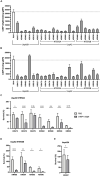
Moraxella catarrhalis is a human-specific commensal of the respiratory tract and an opportunistic pathogen. It is one of the leading cause of otitis media in children and of acute exacerbations in patients with chronic obstructive pulmonary disease, resulting in significant morbidity and economic burden. Vaccines and new immunotherapeutic strategies to treat this emerging pathogen are needed. Complement is a key component of innate immunity that mediates the detection, response, and subsequent elimination of invading pathogens. Many pathogens including M. catarrhalis have evolved complement evasion mechanisms, which include the binding of human complement inhibitors such as C4b-binding protein (C4BP) and Factor H (FH). Inhibiting C4BP and FH acquisition by M. catarrhalis may provide a novel therapeutic avenue to treat infections. To achieve this, we created two chimeric proteins that combined the Moraxella-binding domains of C4BP and FH fused to human immunoglobulin Fcs: C4BP domains 1 and 2 and FH domains 6 and 7 fused to IgM and IgG Fc, respectively. As expected, FH6-7/IgG displaced FH from the bacterial surface while simultaneously activating complement via Fc-C1q interactions, together increasing pathogen elimination. C4BP1-2/IgM also increased serum killing of the bacteria through enhanced complement deposition, but did not displace C4BP from the surface of M. catarrhalis. These Fc fusion proteins could act as anti-infective immunotherapies. Many microbes bind the complement inhibitors C4BP and FH through the same domains as M. catarrhalis, therefore these Fc fusion proteins may be promising candidates as adjunctive therapy against many different drug-resistant pathogens.
Keywords: Moraxella catarrhalis; antibacterial; complement; fusion proteins; pathogen.
Copyright © 2020 Laabei, Colineau, Bettoni, Maziarz, Ermert, Riesbeck, Ram and Blom.
Figures
Similar articles
-
Fusion protein comprising factor H domains 6 and 7 and human IgG1 Fc as an antibacterial immunotherapeutic.Clin Vaccine Immunol. 2014 Oct;21(10):1452-9. doi: 10.1128/CVI.00444-14. Epub 2014 Aug 20. Clin Vaccine Immunol. 2014. PMID: 25143339 Free PMC article.
-
Defining the Binding Region in Factor H to Develop a Therapeutic Factor H-Fc Fusion Protein against Non-Typeable Haemophilus influenzae.Front Cell Infect Microbiol. 2016 Apr 13;6:40. doi: 10.3389/fcimb.2016.00040. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27148489 Free PMC article.
-
Short Leucine-Rich Proteoglycans Modulate Complement Activity and Increase Killing of the Respiratory Pathogen Moraxella catarrhalis.J Immunol. 2018 Nov 1;201(9):2721-2730. doi: 10.4049/jimmunol.1800734. Epub 2018 Sep 28. J Immunol. 2018. PMID: 30266767 Free PMC article.
-
Utilizing complement evasion strategies to design complement-based antibacterial immunotherapeutics: Lessons from the pathogenic Neisseriae.Immunobiology. 2016 Oct;221(10):1110-23. doi: 10.1016/j.imbio.2016.05.016. Epub 2016 Jun 1. Immunobiology. 2016. PMID: 27297292 Free PMC article. Review.
-
Complement evasion by the human respiratory tract pathogens Haemophilus influenzae and Moraxella catarrhalis.FEBS Lett. 2020 Aug;594(16):2586-2597. doi: 10.1002/1873-3468.13758. Epub 2020 Mar 9. FEBS Lett. 2020. PMID: 32053211 Review.
Cited by
-
Recruitment of C4b-binding protein is not a complement evasion strategy employed by Staphylococcus aureus.Microbiology (Reading). 2023 Sep;169(9):001391. doi: 10.1099/mic.0.001391. Microbiology (Reading). 2023. PMID: 37668351 Free PMC article.
-
Nontypeable Haemophilus influenzae P5 Binds Human C4b-Binding Protein, Promoting Serum Resistance.J Immunol. 2021 Sep 15;207(6):1566-1577. doi: 10.4049/jimmunol.2100105. Epub 2021 Aug 25. J Immunol. 2021. PMID: 34433620 Free PMC article.
-
Diverse Functions of C4b-Binding Protein in Health and Disease.J Immunol. 2023 Nov 15;211(10):1443-1449. doi: 10.4049/jimmunol.2300333. J Immunol. 2023. PMID: 37931209 Free PMC article. Review.
-
Proteomics and Microbiota Conjoint Analysis in the Nasal Mucus: Revelation of Differences in Immunological Function in Manis javanica and Manis pentadactyla.Animals (Basel). 2024 Sep 14;14(18):2683. doi: 10.3390/ani14182683. Animals (Basel). 2024. PMID: 39335272 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

