Immunodiversity of the Arabidopsis ZAR1 NLR Is Conveyed by Receptor-Like Cytoplasmic Kinase Sensors
- PMID: 32983191
- PMCID: PMC7475706
- DOI: 10.3389/fpls.2020.01290
Immunodiversity of the Arabidopsis ZAR1 NLR Is Conveyed by Receptor-Like Cytoplasmic Kinase Sensors
Abstract
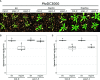
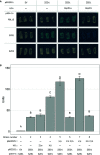
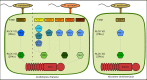
The Arabidopsis nucleotide-binding leucine-rich repeat protein ZAR1 can recognize at least six distinct families of pathogenic effector proteins to mount an effector-triggered immune response. This remarkable immunodiversity appears to be conveyed by receptor-like cytoplasmic kinase (RLCK) complexes, which associate with ZAR1 to sense several effector-induced kinase perturbations. Here we show that the recently identified ZAR1-mediated immune responses against the HopX1, HopO1, and HopBA1 effector families of Pseudomonas syringae rely on an expanded diversity of RLCK sensors. We show that individual sensors can recognize distinct effector families, thereby contributing to the expanded surveillance potential of ZAR1 and supporting its role as a guardian of the plant kinome.
Keywords: Arabidopsis; Pseudomonas syringae; ZAR1; ZED/ZRK; effector-triggered immunity; immunodiversity; receptor-like cytoplasmic kinase (RLCK).
Copyright © 2020 Martel, Laflamme, Seto, Bastedo, Dillon, Almeida, Guttman and Desveaux.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

