Vascular NRP2 triggers PNET angiogenesis by activating the SSH1-cofilin axis
- PMID: 32983407
- PMCID: PMC7509939
- DOI: 10.1186/s13578-020-00472-6
Vascular NRP2 triggers PNET angiogenesis by activating the SSH1-cofilin axis
Abstract
Background: Angiogenesis is a critical step in the growth of pancreatic neuroendocrine tumors (PNETs) and may be a selective target for PNET therapy. However, PNETs are robustly resistant to current anti-angiogenic therapies that primarily target the VEGFR pathway. Thus, the mechanism of PNET angiogenesis urgently needs to be clarified.
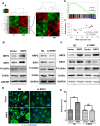
Methods: Dataset analysis was used to identify angiogenesis-related genes in PNETs. Immunohistochemistry was performed to determine the relationship among Neuropilin 2 (NRP2), VEGFR2 and CD31. Cell proliferation, wound-healing and tube formation assays were performed to clarify the function of NRP2 in angiogenesis. The mechanism involved in NRP2-induced angiogenesis was detected by constructing plasmids with mutant variants and performing Western blot, and immunofluorescence assays. A mouse model was used to evaluate the effect of the NRP2 antibody in vivo, and clinical data were collected from patient records to verify the association between NRP2 and patient prognosis.
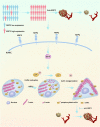
Results: NRP2, a VEGFR2 co-receptor, was positively correlated with vascularity but not with VEGFR2 in PNET tissues. NRP2 promoted the migration of human umbilical vein endothelial cells (HUVECs) cultured in the presence of conditioned medium PNET cells via a VEGF/VEGFR2-independent pathway. Moreover, NRP2 induced F-actin polymerization by activating the actin-binding protein cofilin. Cofilin phosphatase slingshot-1 (SSH1) was highly expressed in NRP2-activating cofilin, and silencing SSH1 ameliorated NRP2-activated HUVEC migration and F-actin polymerization. Furthermore, blocking NRP2 in vivo suppressed PNET angiogenesis and tumor growth. Finally, elevated NRP2 expression was associated with poor prognosis in PNET patients.
Conclusion: Vascular NRP2 promotes PNET angiogenesis by activating the SSH1/cofilin/actin axis. Our findings demonstrate that NRP2 is an important regulator of angiogenesis and a potential therapeutic target of anti-angiogenesis therapy for PNET.
Keywords: Angiogenesis; Cofilin; Neuropilin 2; Pancreatic neuroendocrine tumor; SSH1.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures





References
-
- Mitry E, Walter T, Baudin E, Kurtz JE, Ruszniewski P, Dominguez-Tinajero S, et al. Bevacizumab plus capecitabine in patients with progressive advanced well-differentiated neuroendocrine tumors of the gastro-intestinal (GI-NETs) tract (BETTER trial)–a phase II non-randomised trial. Eur J Cancer. 2014;50:3107–3115. doi: 10.1016/j.ejca.2014.10.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

