Severe combined cardiac and neuromuscular toxicity from immune checkpoint blockade: an institutional case series
- PMID: 32983574
- PMCID: PMC7513476
- DOI: 10.1186/s40959-020-00076-6
Severe combined cardiac and neuromuscular toxicity from immune checkpoint blockade: an institutional case series
Abstract
Background: Immune checkpoint inhibition is part of standard systemic management for many advanced malignancies. Toxicities from this treatment approach are unpredictable, though usually reversible with management per established guidelines. Some patients suffer major morbidity and treatment-related mortality from these agents in an unpredictable manner. Cardiac and neurologic complications are rare, but can result in serious clinical consequences.
Methods: We describe the presentation, management, and outcomes of eight sequential cases of combined cardiac and neurologic toxicities resulting in severe illness and demonstrating lack of rapid response to immunosuppression.
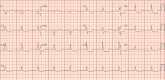
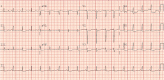
Results: Our cohort consisted of six males and two females with an average age of 73.5 years (61-89 years). There were four patients with melanoma, and one patient each with urothelial carcinoma, renal cell carcinoma, breast cancer, and non-small cell lung cancer. Four patients received combination immunotherapy and four patients received monotherapy. The median time to presentation from treatment initiation was 27 days (11-132 days). All patients had a cardiovascular and neurologic toxicity, and most had hepatitis and myositis. The cardiac signs and symptoms were the prominent initial features of the clinical presentation. Each patient was managed by a multidisciplinary team and received a range of immunosuppressive agents. All patients died as a consequence of the immune related adverse events.
Conclusions: The evaluation of patients with cardiac adverse events from immunotherapy, should include assessment of overlapping toxicities such as myasthenia gravis and myositis. Providers should be aware of the potential for an extended duration of disability and slow improvement for certain toxicities as these expectations may factor prominently in goals of care decisions.
Keywords: Combination immunotherapy; Immune related adverse events; Myasthenia gravis; Myocarditis; Myositis.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
