Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis
- PMID: 32983575
- PMCID: PMC7486930
- DOI: 10.1038/s41536-020-00100-4
Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis
Abstract
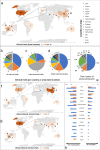
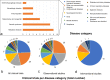
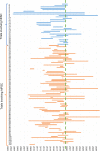
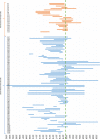
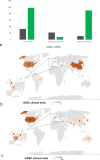
Pluripotent stem cells (PSCs) hold great potential for novel therapeutic approaches to regenerate or replace functionally impaired tissues. Since the introduction of the induced pluripotent stem cell technology in 2006, the number of scientific publications on this topic has constantly been increasing. However, so far no therapy based on PSCs has found its way into routine clinical use. In this study, we examined research trends related to clinical trials involving PSCs based on data obtained from ClinicalTrials.gov, the ICTRP database from the World Health Organization, as well as from a search of all individual databases that are included in the ICTRP using a multistep search algorithm. Following a stringent inclusion/exclusion procedure 131 studies remained that could be classified as clinical trials involving PSCs. The magnitude of these studies (77.1%) was observational, which implies that no cells were transplanted into patients, and only a minority of studies (22.9%) were of an interventional study type. The number of clinical trials involving induced pluripotent stem cells (iPSCs, 74.8%) was substantially higher than the one involving embryonic stem cells (ESCs, 25.2%). However, the picture changes completely when focusing on interventional studies, where in the majority (73.3%) of cases ESCs were used. Interestingly, also the study duration was significantly shorter for interventional versus observational trials (p = 0.002). When focusing on the geographical study regions, it became obvious that the greatest part of all observational trials was performed in the USA (41.6%) and in France (16.8%), while the magnitude of interventional studies was performed in Asian countries (China 36.7%, Japan 13.3%, South Korea 10.0%) and in the field of ophthalmology. In summary, these results indicate that only a limited number of trials were focusing on the actual transplantation of PSCs into patients in a rather narrow field of diagnoses. The future will tell us, if the iPSC technology will ultimately overcome the current challenges and will finally make its way into routine clinical use.
Keywords: Embryonic stem cells; Induced pluripotent stem cells.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
References
-
- Kimbrel EA, Lanza R. Pluripotent stem cells: the last 10 years. Regen. Med. 2016;11:831–847. - PubMed
-
- da Cruz L, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018;36:328–337. - PubMed
-
- Schwartz SD, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

