MEK Inhibitor Augments Antitumor Activity of B7-H3-Redirected Bispecific Antibody
- PMID: 32984002
- PMCID: PMC7477310
- DOI: 10.3389/fonc.2020.01527
MEK Inhibitor Augments Antitumor Activity of B7-H3-Redirected Bispecific Antibody
Abstract
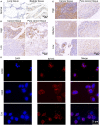
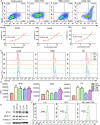
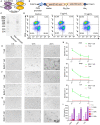
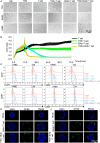
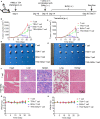
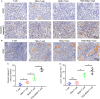
Targeting cancer antigens by T cell-engaging bispecific antibody (BiAb) or chimeric antigen receptor T cell therapy has achieved successes in hematological cancers, but attempts to use it to fight solid cancers have been disappointing, in part due to antigen escape. MEK inhibitor had limited activity as a single agent, but enhanced antitumor activity when combined with other therapies, such as targeted drugs or immunotherapy agents. This study aimed to analyze the expression of B7-H3 in non-small-cell lung cancer (NSCLC) and bladder cancer (BC) and to evaluate the combinatorial antitumor effect of B7-H3 × CD3 BiAb with MEK inhibitor trametinib. We found B7-H3 was highly expressed in NSCLC and BC compared with normal samples and its increased expression was associated with poor prognosis. Treatment with trametinib alone could induce apoptosis in tumor cell, while has no effect on T cell proliferation, and a noticeable elevation of B7-H3 expression in tumor cells was also observed following treatment. B7-H3 × CD3 BiAb specifically and efficiently redirected their cytotoxicity against B7-H3 overexpressing tumor cells both in vitro and in xenograft mouse models. While trametinib treatment alone affected tumor growth, the combined therapy increased T cell infiltration and significantly suppressed tumor growth. Together, these data suggest that combination therapy with B7-H3 × CD3 BiAb and MEK inhibitor may serve as a new therapeutic strategy in the future clinical practice for the treatment of NSCLC and BC.
Keywords: B7-H3; bispecific antibody; bladder cancer; immunotherapy; non-small-cell lung cancer; trametinib.
Copyright © 2020 Li, Huang, Zhang, Feng, Wang, Tang, Zhong, Hu, Guo, Zhou, Guo, Xu, Yang and Tong.
Figures

Similar articles
-
Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes.J Hematol Oncol. 2021 Jan 29;14(1):21. doi: 10.1186/s13045-020-01024-8. J Hematol Oncol. 2021. PMID: 33514401 Free PMC article.
-
A Novel Anti-B7-H3 × Anti-CD3 Bispecific Antibody with Potent Antitumor Activity.Life (Basel). 2022 Jan 21;12(2):157. doi: 10.3390/life12020157. Life (Basel). 2022. PMID: 35207448 Free PMC article.
-
Bispecific anti-CD3 x anti-B7-H3 antibody mediates T cell cytotoxic ability to human melanoma in vitro and in vivo.Invest New Drugs. 2019 Oct;37(5):1036-1043. doi: 10.1007/s10637-018-00719-7. Epub 2019 Feb 1. Invest New Drugs. 2019. PMID: 30706335
-
B7-H3-targeted CAR-T cell therapy for solid tumors.Int Rev Immunol. 2022;41(6):625-637. doi: 10.1080/08830185.2022.2102619. Epub 2022 Jul 20. Int Rev Immunol. 2022. PMID: 35855615 Review.
-
Targeting B7-H3-A Novel Strategy for the Design of Anticancer Agents for Extracranial Pediatric Solid Tumors Treatment.Molecules. 2023 Apr 11;28(8):3356. doi: 10.3390/molecules28083356. Molecules. 2023. PMID: 37110590 Free PMC article. Review.
Cited by
-
The genomic landscape of pediatric renal cell carcinomas.iScience. 2022 Mar 26;25(4):104167. doi: 10.1016/j.isci.2022.104167. eCollection 2022 Apr 15. iScience. 2022. PMID: 35445187 Free PMC article.
-
Treating Bladder Cancer: Engineering of Current and Next Generation Antibody-, Fusion Protein-, mRNA-, Cell- and Viral-Based Therapeutics.Front Oncol. 2021 May 27;11:672262. doi: 10.3389/fonc.2021.672262. eCollection 2021. Front Oncol. 2021. PMID: 34123841 Free PMC article. Review.
-
Crosstalk between the B7/CD28 and EGFR pathways: Mechanisms and therapeutic opportunities.Genes Dis. 2021 Sep 20;9(5):1181-1193. doi: 10.1016/j.gendis.2021.08.009. eCollection 2022 Sep. Genes Dis. 2021. PMID: 35873032 Free PMC article. Review.
-
Cell Therapies in Bladder Cancer Management.Int J Mol Sci. 2021 Mar 10;22(6):2818. doi: 10.3390/ijms22062818. Int J Mol Sci. 2021. PMID: 33802203 Free PMC article. Review.
-
Interplay between B7-H3 and HLA class I in the clinical course of pancreatic ductal adenocarcinoma.Cancer Lett. 2024 Apr 10;587:216713. doi: 10.1016/j.canlet.2024.216713. Epub 2024 Feb 14. Cancer Lett. 2024. PMID: 38364961 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

