Abnormal Ferroptosis in Myelodysplastic Syndrome
- PMID: 32984038
- PMCID: PMC7492296
- DOI: 10.3389/fonc.2020.01656
Abnormal Ferroptosis in Myelodysplastic Syndrome
Abstract
Background: Ferroptosis is a form of iron-dependent non-apoptotic cell death, with characteristics of loss of the activity of the lipid repair enzyme, glutathione (GSH) peroxidase 4 (GPX4), and accumulation of lethal reactive lipid oxygen species. The mechanism of ferroptosis in myelodysplastic syndrome (MDS) is unclear.
Methods: Cell viability assay, reactive oxygen species (ROS) assay, GSH assay, and GPX activity assay were performed to study the regulation of ferroptosis in MDS cells obtained from MDS patients, the iron overload model mice, and cell lines.
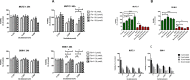
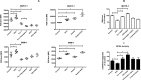
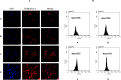
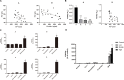
Results: The growth-inhibitory effect of decitabine could be partially reversed by ferrostatin-1 and iron-chelating agent [desferrioxamine (DFO)] in MDS cell lines. Erastin could increase the cytotoxicity of decitabine on MDS cells. The level of GSH and the activity of GPX4 decreased, whereas the ROS level increased in MDS cells upon treatment with decitabine, which could be reversed by ferrostatin-1. The concentration of hemoglobin in peripheral blood of iron overload mice was negatively correlated with intracellular Fe2+ level and ferritin concentration. Iron overload (IO) led to decreased viability of bone marrow mononuclear cells (BMMNCs), which was negatively correlated with intracellular Fe2+ level. Ferrostatin-1 partially reversed the decline of cell viability in IO groups. The level of GSH and the activity of GPX4 decreased, whereas the ROS level increased in BMMNCs of IO mice. DFO could increase the level of GSH. Ferrostatin-1 and DFO could increase the GPX4 activity of BMMNCs in IO mice. Ferrostatin-1 could significantly reverse the growth-inhibitory effect of decitabine in MDS patients. Decitabine could significantly increase the ROS level in MDS groups, which could be inhibited by ferrostatin-1 or promoted by erastin. Ferrostatin-1 could significantly reverse the inhibitory effect of decitabine on GSH levels in MDS patients. Erastin combined with decitabine could further reduce the GSH level. Erastin could further decrease the activity of GPX4 compared with the decitabine group.
Conclusion: Ferroptosis may account for the main mechanisms of how decitabine induced death of MDS cells. Decitabine-induced ROS raise leads to ferroptosis in MDS cells by decreasing GSH level and GPX4 activity.
Keywords: apoptosis; decitabine; ferroptosis; myelodysplastic syndrome; pyroptosis.
Copyright © 2020 Lv, Niu, Yue, Liu, Yang, Liu, Jiang, Dong, Shao, Xing and Wang.
Figures

Similar articles
-
Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms.DNA Cell Biol. 2021 Feb;40(2):172-183. doi: 10.1089/dna.2020.5730. Epub 2020 Dec 22. DNA Cell Biol. 2021. PMID: 33351681
-
A new therapeutic perspective: Erastin inhibits tumor progression by driving ferroptosis in myelodysplastic syndromes.J Investig Med. 2024 Jun;72(5):414-424. doi: 10.1177/10815589241246541. Epub 2024 May 11. J Investig Med. 2024. PMID: 38557364
-
Effect of iron overload on endothelial cell calcification and its mechanism.Ann Transl Med. 2021 Nov;9(22):1658. doi: 10.21037/atm-21-5666. Ann Transl Med. 2021. PMID: 34988167 Free PMC article.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
-
System Xc -/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy.Front Pharmacol. 2022 Aug 29;13:910292. doi: 10.3389/fphar.2022.910292. eCollection 2022. Front Pharmacol. 2022. PMID: 36105219 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Ferroptosis and Its Role in Viral Pathogenesis.Viruses. 2023 Dec 1;15(12):2373. doi: 10.3390/v15122373. Viruses. 2023. PMID: 38140616 Free PMC article. Review.
-
Ferroptosis: mechanisms and advances in ocular diseases.Mol Cell Biochem. 2023 Sep;478(9):2081-2095. doi: 10.1007/s11010-022-04644-5. Epub 2023 Jan 8. Mol Cell Biochem. 2023. PMID: 36617346 Review.
-
The Clinical Significance of Iron Overload and Iron Metabolism in Myelodysplastic Syndrome and Acute Myeloid Leukemia.Front Immunol. 2021 Feb 19;11:627662. doi: 10.3389/fimmu.2020.627662. eCollection 2020. Front Immunol. 2021. PMID: 33679722 Free PMC article. Review.
-
Exploiting cell death and tumor immunity in cancer therapy: challenges and future directions.Front Cell Dev Biol. 2024 Jun 3;12:1416115. doi: 10.3389/fcell.2024.1416115. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38887519 Free PMC article. Review.
-
Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology.Eur J Med Res. 2024 Apr 9;29(1):224. doi: 10.1186/s40001-024-01822-7. Eur J Med Res. 2024. PMID: 38594732 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

