Extracellular Vesicles Reflect the Efficacy of Wheatgrass Juice Supplement in Colon Cancer Patients During Adjuvant Chemotherapy
- PMID: 32984039
- PMCID: PMC7479215
- DOI: 10.3389/fonc.2020.01659
Extracellular Vesicles Reflect the Efficacy of Wheatgrass Juice Supplement in Colon Cancer Patients During Adjuvant Chemotherapy
Abstract
Introduction: Colorectal cancer (CC) is the third most common type of cancer, accounting for 10% of all cancer cases. Adjuvant chemotherapy is recommended in stages II-III CC. Wheatgrass juice (WGJ) from wheat seeds has high nutritional values, may induce synergistic benefits to chemotherapy and may attenuate chemotherapy-related side effects. Extracellular vesicles (EVs) are subcellular membrane blebs. EVs include exosomes (generated in the endosome, in size <150 nm) and microvesicles (shed from the plasma cell membrane) provide information on their parental cells and play a role in intercellular communication. We aimed to elucidate the effects of chemotherapy administration with supportive treatment of WGJ on CC patients' EVs characteristics.
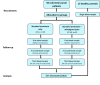
Methods: EVs were isolated from the blood samples of 15 healthy controls (HCs) and 50 CC patients post-surgery, treated by chemotherapy, with or without additional daily WGJ. Blood samples were taken before, during, and at the end of chemotherapy. EVs were characterized by size, concentration, membrane antigens and cytokine content using nanoparticle-tracking analysis, western blot, flow cytometry, and protein array methods.
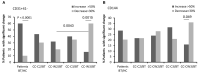
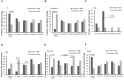
Results: EVs were found to be similar by size and concentration with reduced levels of exosome markers (CD81) on samples at the end of combined treatment (chemotherapy and WGJ). Higher levels of endothelial EVs, which may indicate impairment of the vascular endothelial cells during treatment, were found in CC patients treated by chemotherapy only compared to those with chemotherapy and daily WGJ. Also, EVs thrombogenicity was lower in patients added WGJ compared to patients who had only chemotherapy (levels of tissue factor p = 0.029 and endothelial protein C receptor p = 0.005). Following treatments, levels of vascular endothelial growth factor receptors (VEGFR-1) and the majority of growth-factors/pro-inflammatory cytokines were higher in EVs of patients treated by chemotherapy only than in EVs obtained from patients with the combined treatment.
Conclusion: Daily consumption of WGJ during chemotherapy may reduce vascular damage and chemotherapy-related thrombogenicity, growth factors and cytokines, as reflected by the characteristics of patient's EVs.
Keywords: adjuvant chemotherapy; colon cancer; cytokines; extracellular vesicles; thrombogenicity; wheatgrass juice.
Copyright © 2020 Avisar, Cohen, Brenner, Bronshtein, Machluf, Bar-Sela and Aharon.
Figures

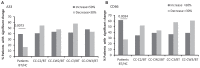
Similar articles
-
Wheatgrass Juice Administration and Immune Measures during Adjuvant Chemotherapy in Colon Cancer Patients: Preliminary Results.Pharmaceuticals (Basel). 2020 Jun 23;13(6):129. doi: 10.3390/ph13060129. Pharmaceuticals (Basel). 2020. PMID: 32585974 Free PMC article.
-
Chemotherapy administration to breast cancer patients affects extracellular vesicles thrombogenicity and function.Oncotarget. 2017 Jun 28;8(38):63265-63280. doi: 10.18632/oncotarget.18792. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28968987 Free PMC article.
-
Circulating blood extracellular vesicles as a tool to assess endothelial injury and chemotherapy toxicity in adjuvant cancer patients.PLoS One. 2020 Oct 27;15(10):e0240994. doi: 10.1371/journal.pone.0240994. eCollection 2020. PLoS One. 2020. PMID: 33108394 Free PMC article.
-
Intercellular Communication by Vascular Endothelial Cell-Derived Extracellular Vesicles and Their MicroRNAs in Respiratory Diseases.Front Mol Biosci. 2021 Jan 29;7:619697. doi: 10.3389/fmolb.2020.619697. eCollection 2020. Front Mol Biosci. 2021. PMID: 33614707 Free PMC article. Review.
-
Therapeutic Potential of Wheatgrass Juice: A Comprehensive Narrative Review.J Pharm Bioallied Sci. 2025 May;17(Suppl 1):S152-S154. doi: 10.4103/jpbs.jpbs_179_25. Epub 2025 Apr 21. J Pharm Bioallied Sci. 2025. PMID: 40511096 Free PMC article. Review.
Cited by
-
Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis.Front Oncol. 2022 Aug 31;12:966981. doi: 10.3389/fonc.2022.966981. eCollection 2022. Front Oncol. 2022. PMID: 36119470 Free PMC article. Review.
-
Capacity Building of a Self-Reliant Model Community for Cholangiocarcinoma Prevention by Producing Fruit and Vegetable Juice Products in a High-Risk Area of Thailand.Asian Pac J Cancer Prev. 2023 Feb 1;24(2):725-731. doi: 10.31557/APJCP.2023.24.2.725. Asian Pac J Cancer Prev. 2023. PMID: 36853325 Free PMC article.
-
Extracellular Vesicles of COVID-19 Patients Reflect Inflammation, Thrombogenicity, and Disease Severity.Int J Mol Sci. 2023 Mar 21;24(6):5918. doi: 10.3390/ijms24065918. Int J Mol Sci. 2023. PMID: 36982991 Free PMC article.
-
Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies.J Hematol Oncol. 2024 Nov 5;17(1):105. doi: 10.1186/s13045-024-01625-7. J Hematol Oncol. 2024. PMID: 39501358 Free PMC article. Review.
-
Recent advances in targeted drug delivery systems for resistant colorectal cancer.Cancer Cell Int. 2022 May 19;22(1):196. doi: 10.1186/s12935-022-02605-y. Cancer Cell Int. 2022. PMID: 35590367 Free PMC article. Review.
References
-
- Bar-Sela G, Cohen M, Ben-Arye E, Epelbaum R. The medical use of wheatgrass: review of the gap between basic and clinical applications. Mini Rev Med Chem. (2015) 15:1002–10. - PubMed
LinkOut - more resources
Full Text Sources

