Topic Application of the Probiotic Streptococcus dentisani Improves Clinical and Microbiological Parameters Associated With Oral Health
- PMID: 32984080
- PMCID: PMC7488176
- DOI: 10.3389/fcimb.2020.00465
Topic Application of the Probiotic Streptococcus dentisani Improves Clinical and Microbiological Parameters Associated With Oral Health
Abstract
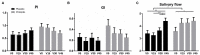
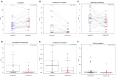
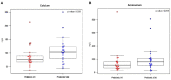
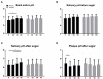
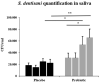
Streptococcus dentisani 7746, isolated from dental plaque of caries-free individuals, has been shown to have several beneficial effects in vitro which could contribute to promote oral health, including an antimicrobial activity against oral pathogens by the production of bacteriocins and a pH buffering capacity through ammonia production. Previous work has shown that S. dentisani was able to colonize the oral cavity for 2-4 weeks after application. The aim of the present work was to evaluate its clinical efficacy by a randomized, double-blind, placebo-controlled parallel group study. Fifty nine volunteers were enrolled in the study and randomly assigned to a treatment or placebo group. The treatment consisted of a bucco-adhesive gel application (2.5 109 cfu/dose) with a dental splint for 5 min every 48 h, for a period of 1 month (i.e., 14 doses). Dental plaque and saliva samples were collected at baseline, 15 and 30 days after first application, and 15 days after the end of treatment. At baseline, there was a significant correlation between S. dentisani levels and frequency of toothbrushing. Salivary flow, a major factor influencing oral health, was significantly higher in the probiotic group at day 15 compared with the placebo (4.4 and 3.4 ml/5 min, respectively). In the probiotic group, there was a decrease in the amount of dental plaque and in gingival inflammation, but no differences were observed in the placebo group. The probiotic group showed a significant increase in the levels of salivary ammonia and calcium. Finally, Illumina sequencing of plaque samples showed a beneficial shift in bacterial composition at day 30 relative to baseline, with a reduction of several cariogenic organisms and the key players in plaque formation, probably as a result of bacteriocins production. Only 58% of the participants in the probiotic group showed increased plaque levels of S. dentisani at day 30 and 71% by day 45, indicating that the benefits of S. dentisani application could be augmented by improving colonization efficiency. In conclusion, the application of S. dentisani 7746 improved several clinical and microbiological parameters associated with oral health, supporting its use as a probiotic to prevent tooth decay.
Keywords: bacteriocin; dental caries; dental plaque; probiotic; salivary flow.
Copyright © 2020 Ferrer, López-López, Nicolescu, Perez-Vilaplana, Boix-Amorós, Dzidic, Garcia, Artacho, Llena and Mira.
Figures

References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

