Survival Outcomes for Yttrium-90 Transarterial Radioembolization With and Without Sorafenib for Unresectable Hepatocellular Carcinoma Patients
- PMID: 32984089
- PMCID: PMC7500841
- DOI: 10.2147/JHC.S248314
Survival Outcomes for Yttrium-90 Transarterial Radioembolization With and Without Sorafenib for Unresectable Hepatocellular Carcinoma Patients
Abstract
Purpose: To assess the overall survival (OS) and progression-free survival (PFS) of unresectable hepatocellular carcinoma (HCC) patients undergoing yttrium-90 glass-microsphere transarterial radioembolization (TARE) with and without concurrent sorafenib.
Methods: OS and PFS were analyzed in 55 patients with an intrahepatic tumor (IHT) ≤50% without advanced or aggressive disease features (ADFs), which was referred to presence of infiltrative/ill-defined HCC, macrovascular invasion, or extrahepatic disease treated with only TARE (TARE_alone) and in 74 patients with IHT ≤50% with ADFs or IHT >50% treated with TARE and sorafenib (TARE_sorafenib). Prognostic factors for OS and PFS were identified using univariate and multivariate analyses.
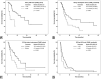
Results: Median OS and PFS of TARE_alone patients were 21.6 (95% CI 6.1-37.1) and 9.1(95% CI 5.2-13.0) months, respectively, and for TARE_sorafenib patients 12.4 (95% CI 9.1-15.6) and 5.1 (95% CI 2.6-7.5) months, respectively. Better OS was associated with serum AFP <400 (HR 0.27, p=0.02) in TARE_alone, and IHT ≤50% (HR 0.39, p=0.004) and AFP <400 (HR 0.5, p=0.027) in TARE_sorafenib. Unilobar involvement (HR 0.43, p=0.029) and AFP <400 ng/mL (HR 0.52, p=0.015) correlated with better PFS in TARE_alone and TARE_sorafenib, respectively. Adverse events (AEs) were more frequent in TARE_sorafenib than TARE_alone (92.4 vs 80.3%), but only 9.3% were grade 3 or higher AEs.
Conclusion: TARE_alone provided the most prominent survival benefit in IHT ≤50%-without ADF patients who had unilobar HCC and serum AFP <400 ng/mL. TARE and sorafenib yielded the best outcomes in patients with IHT ≤50% and serum AFP <400 ng/mL, with some additional grade 1-2 AEs compared to TARE only.
Keywords: 90Y; TheraSphere; adverse events; prognostic factors; selective internal radiation therapy.
© 2020 Teyateeti et al.
Conflict of interest statement
AM has received research grants from BTG International, Sirtex Medical, and ABK Medical and serves as a consultant for BTG, Sirtex, and Boston Scientific. JDK has received a research grant from Cynvenio Biosystems and is on a speaker bureau of Angiodynamics. He reports personal fees from BTG and Argon, and serves on an advisory board for Boston Scientific. BC serves on an advisory board for Advanced Accelerator Applications and Clovis Oncology. BCO. has received a research grant from Siemens Healthineers and serves as a consultant for Koo Foundation. SCK has received research grants from BTG International and GE Healthcare and serves as a consultant for BTG International, Terumo Medical, Sirtex Medical, and ABK Biomedical. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Disease control and failure patterns of unresectable hepatocellular carcinoma following transarterial radioembolization with yttrium-90 microspheres and with/without sorafenib.World J Gastroenterol. 2021 Dec 21;27(47):8166-8181. doi: 10.3748/wjg.v27.i47.8166. World J Gastroenterol. 2021. PMID: 35068861 Free PMC article.
-
Transarterial Radioembolization Versus Atezolizumab-Bevacizumab in Unresectable Hepatocellular Carcinoma: A Matching-Adjusted Indirect Comparison of Time to Deterioration in Quality of Life.Adv Ther. 2022 May;39(5):2035-2051. doi: 10.1007/s12325-022-02099-0. Epub 2022 Mar 12. Adv Ther. 2022. PMID: 35279814 Free PMC article.
-
18F-FDG PET/CT predicts survival after 90Y transarterial radioembolization in unresectable hepatocellular carcinoma.Eur J Nucl Med Mol Imaging. 2017 Jul;44(7):1215-1222. doi: 10.1007/s00259-017-3653-0. Epub 2017 Feb 23. Eur J Nucl Med Mol Imaging. 2017. PMID: 28233086
-
Transarterial radioembolization using yttrium-90 microspheres in the treatment of hepatocellular carcinoma: a review on clinical utility and developments.J Hepatocell Carcinoma. 2014 Nov 3;1:163-82. doi: 10.2147/JHC.S50472. eCollection 2014. J Hepatocell Carcinoma. 2014. PMID: 27508185 Free PMC article. Review.
-
Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma.Adv Ther. 2016 May;33(5):699-714. doi: 10.1007/s12325-016-0324-7. Epub 2016 Apr 2. Adv Ther. 2016. PMID: 27039186 Free PMC article. Review.
Cited by
-
Improved Survival after Transarterial Radioembolisation for Hepatocellular Carcinoma Gives the Procedure Added Value.J Clin Med. 2022 Dec 16;11(24):7469. doi: 10.3390/jcm11247469. J Clin Med. 2022. PMID: 36556085 Free PMC article.
-
Biochemical Safety of Ablative Yttrium-90 Radioembolization for Hepatocellular Carcinoma as a Function of Percent Liver Treated.J Hepatocell Carcinoma. 2021 Jul 30;8:861-870. doi: 10.2147/JHC.S319215. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 34368021 Free PMC article.
-
The American Brachytherapy Society consensus statement for permanent implant brachytherapy using Yttrium-90 microsphere radioembolization for liver tumors.Brachytherapy. 2022 Sep-Oct;21(5):569-591. doi: 10.1016/j.brachy.2022.04.004. Epub 2022 May 20. Brachytherapy. 2022. PMID: 35599080 Free PMC article.
-
Efficacy of Yttrium-90 Transarterial Radioembolisation in Advanced Hepatocellular Carcinoma: An Experience With Hybrid Angio-Computed Tomography and Glass Microspheres.J Clin Exp Hepatol. 2024 May-Jun;14(3):101342. doi: 10.1016/j.jceh.2023.101342. Epub 2023 Dec 26. J Clin Exp Hepatol. 2024. PMID: 38283702 Free PMC article.
-
Yttrium-90 Radioembolization Dosimetry: Dose Considerations, Optimization, and Tips.Semin Intervent Radiol. 2024 Mar 14;41(1):63-78. doi: 10.1055/s-0044-1779715. eCollection 2024 Feb. Semin Intervent Radiol. 2024. PMID: 38495257 Free PMC article. Review. No abstract available.
References
-
- Liver cancer [homepage on the Internet]. The global cancer observatory cancer fact sheets; 2018. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. Accessed May2, 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

