Calcium Dynamics in Astrocytes During Cell Injury
- PMID: 32984268
- PMCID: PMC7481337
- DOI: 10.3389/fbioe.2020.00912
Calcium Dynamics in Astrocytes During Cell Injury
Abstract
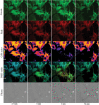
The changes in intracellular calcium concentration ([Ca2+]) following laser-induced cell injury in nearby cells were studied in primary mouse astrocytes selectively expressing the Ca2+ sensitive GFAP-Cre Salsa6f fluorescent tandem protein, in an Ast1 astrocyte cell line, and in primary mouse astrocytes loaded with Fluo4. Astrocytes in these three systems exhibit distinct changes in [Ca2+] following induced death of nearby cells. Changes in [Ca2+] appear to result from release of Ca2+ from intracellular organelles, as opposed to influx from the external medium. Salsa6f expressing astrocytes displayed dynamic Ca2+ changes throughout the phagocytic response, including lamellae protrusion, cytosolic signaling during vesicle formation, vesicle maturation, and vesicle tract formation. Our results demonstrate local changes in [Ca2+] are involved in the process of phagocytosis in astrocytes responding to cell corpses and/or debris.
Keywords: Salsa6f; astrocyte; calcium; laser ablation; laser nanosurgery; phagocytosis; photolysis.
Copyright © 2020 Wakida, Gomez-Godinez, Li, Nguyen, Kim, Dynes, Othy, Lau, Ding, Shi, Carmona, Thompson, Cahalan and Berns.
Figures











References
-
- Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28 264–278. 10.1523/jneurosci.4178-07.2008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

