Cobetia sp. Bacteria, Which Are Capable of Utilizing Alginate or Waste Laminaria sp. for Poly(3-Hydroxybutyrate) Synthesis, Isolated From a Marine Environment
- PMID: 32984275
- PMCID: PMC7479843
- DOI: 10.3389/fbioe.2020.00974
Cobetia sp. Bacteria, Which Are Capable of Utilizing Alginate or Waste Laminaria sp. for Poly(3-Hydroxybutyrate) Synthesis, Isolated From a Marine Environment
Abstract
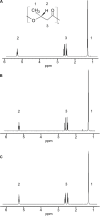
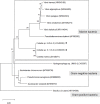
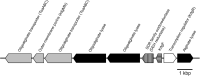
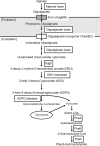
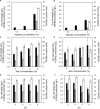
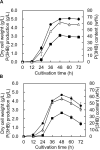
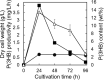
We isolated the Cobetia sp. strains IU 180733JP01 (5-11-6-3) and 190790JP01 (5-25-4-2) from seaweeds and showed that both strains accumulate poly(3-hydroxybutyrate) [P(3HB)] homopolymer in a nitrogen-limiting mineral salt medium containing alginate as a sole carbon source. Genome sequence analysis of the isolated strains showed that they have putative genes which encode enzymes relevant to alginate assimilation and P(3HB) synthesis, and the putative alginate-assimilating genes formed a cluster. Investigation of the optimum culture conditions for high accumulation of P(3HB) showed that when the 5-11-6-3 strain was cultured in a nitrogen-limiting mineral salt medium (pH 5.0) containing 6% NaCl and 3% (w/v) alginate as a sole carbon source for 2 days, the P(3HB) content and P(3HB) production reached 62.1 ± 3.4 wt% and 3.11 ± 0.16 g/L, respectively. When the 5-25-4-2 strain was cultured in a nitrogen-limiting mineral salt medium (pH 4.0) containing 5% NaCl and 3% (w/v) alginate for 2 days, the P(3HB) content and P(3HB) production reached 56.9 ± 2.1 wt% and 2.67 ± 0.11 g/L, respectively. Moreover, the 5-11-6-3 strain also produced P(3HB) in a nitrogen-limiting mineral salt medium (pH 5.0) containing 6% NaCl and freeze-dried and crushed waste Laminaria sp., which is classified into brown algae and contains alginate abundantly. The resulting P(3HB) content and P(3HB) productivity were 13.5 ± 0.13 wt% and 3.99 ± 0.15 mg/L/h, respectively. Thus, we demonstrated the potential application of the isolated strains to a simple P(3HB) production process from seaweeds without chemical hydrolysis and enzymatic saccharification.
Keywords: alginic acid brown algae; biopolymer; marine biomass resources; polyhydroxyalkanoate; seaweeds.
Copyright © 2020 Moriya, Takita, Matsumoto, Yamahata, Nishimukai, Miyazaki, Shimoi, Kawai and Yamada.
Figures

Similar articles
-
Utilization of various carbon sources for poly(3-hydroxybutyrate) [P(3HB)] production by Cobetia sp. IU180733JP01 (5-11-6-3) which is capable of producing P(3HB) from alginate and waste seaweed.J Gen Appl Microbiol. 2022 Nov 22;68(4):207-211. doi: 10.2323/jgam.2021.11.002. Epub 2022 Apr 13. J Gen Appl Microbiol. 2022. PMID: 35418539
-
Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution.Microb Cell Fact. 2016 Jun 3;15:95. doi: 10.1186/s12934-016-0495-6. Microb Cell Fact. 2016. PMID: 27260327 Free PMC article.
-
Biosynthesis of Polyhydroxyalkanoate from Steamed Soybean Wastewater by a Recombinant Strain of Pseudomonas sp. 61-3.Bioengineering (Basel). 2017 Aug 8;4(3):68. doi: 10.3390/bioengineering4030068. Bioengineering (Basel). 2017. PMID: 28952548 Free PMC article.
-
Production of poly(3-hydroyxybutylate) by a novel alginolytic bacterium Hydrogenophaga sp. strain UMI-18 using alginate as a sole carbon source.J Biosci Bioeng. 2019 Aug;128(2):203-208. doi: 10.1016/j.jbiosc.2019.02.008. Epub 2019 Mar 7. J Biosci Bioeng. 2019. PMID: 30852124
-
Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review.Int J Biol Macromol. 1999 Jun-Jul;25(1-3):31-6. doi: 10.1016/s0141-8130(99)00012-4. Int J Biol Macromol. 1999. PMID: 10416647 Review.
Cited by
-
In-Depth Genome Characterization and Pan-Genome Analysis of Strain KMM 296, a Producer of Highly Active Alkaline Phosphatase; Proposal for the Reclassification of Cobetia litoralis and Cobetia pacifica as the Later Heterotypic Synonyms of Cobetia amphilecti and Cobetia marina, and Emended Description of the Species Cobetia amphilecti and Cobetia marina.Biomolecules. 2024 Feb 6;14(2):196. doi: 10.3390/biom14020196. Biomolecules. 2024. PMID: 38397433 Free PMC article.
-
Marine-derived biopolymers as potential bioplastics, an eco-friendly alternative.iScience. 2023 Mar 15;26(4):106404. doi: 10.1016/j.isci.2023.106404. eCollection 2023 Apr 21. iScience. 2023. PMID: 37034997 Free PMC article. Review.
-
In silico identification of bacterial seaweed-degrading bioplastic producers.Microb Genom. 2022 Sep;8(9):mgen000866. doi: 10.1099/mgen.0.000866. Microb Genom. 2022. PMID: 36125959 Free PMC article.
-
High natural PHA production from acetate in Cobetia sp. MC34 and Cobetia marina DSM 4741T and in silico analyses of the genus specific PhaC2 polymerase variant.Microb Cell Fact. 2021 Dec 20;20(1):225. doi: 10.1186/s12934-021-01713-0. Microb Cell Fact. 2021. PMID: 34930259 Free PMC article.
-
Bacteria on the foundational kelp in kelp forest ecosystems: Insights from culturing, whole genome sequencing and metabolic assays.Environ Microbiol Rep. 2024 Jun;16(3):e13270. doi: 10.1111/1758-2229.13270. Environ Microbiol Rep. 2024. PMID: 38778582 Free PMC article.
References
-
- Akiyama M., Tsuge T., Doi Y. (2003). Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym. Degrad. Stab. 80 183–194. 10.1016/s0141-3910(02)00400-7 - DOI
-
- Alkotaini B., Koo H., Kim B. S. (2016). Production of polyhydroxyalkanoates by batch and fed-batch cultivations of Bacillus megaterium from acid-treated red algae. Korea. J. Chem. Eng. 33 1669–1673. 10.1007/s11814-015-0293-6 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

