General Analyses of Gene Expression Dependencies on Genetic Burden
- PMID: 32984285
- PMCID: PMC7481379
- DOI: 10.3389/fbioe.2020.01017
General Analyses of Gene Expression Dependencies on Genetic Burden
Abstract
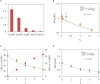
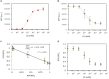
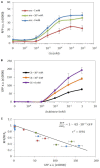
Over the last decade, the combining of newly developed molecular tools for DNA editing with engineering principles has allowed the creation of complex cellular devices, usually based on complex genetic circuits, for many different purposes. However, when the technological evolution of genetic circuitry is compared with previous technologies such as electronic circuitry, clear limitations regarding the technological scalability of genetic circuitry are observed due to the lack of predictability. To overcome this problem, it is necessary to create new theoretical frameworks for designing genetic circuits in a feasible and reliable manner, taking into account those limitations. Among a number of such limitations, the so-called genetic burden is one of the main constraints. Surprisingly, despite its relevance, little attention has been paid to genetic burden, and it is often not considered when designing genetic circuits. In this study, a new general mathematical formalism is presented, describing the effects of genetic burden on gene expression. The mathematical analysis shows that alterations in gene expression due to genetic burden can be qualitatively described independently of the specific genetic features of the system under consideration. The mathematical model was experimentally tested in different genetic circuits. The experimental evidence coincides with the expected behaviors described by the model in complex scenarios. For instance, observed modulations in the expression levels of constitutive genes in response to changes in the levels of external inducers of gene expression that do not directly modulate them, or the emergence of limitations in gene overexpression, can be understood in terms of genetic burden. The present mathematical formalism provides a useful general framework for gene circuit design that will help to advance synthetic biological systems.
Keywords: gene expression; genetic burden; gentic circuits; mathematical model; synthetic biological circuits.
Copyright © 2020 González-Colell and Macía.
Figures
Similar articles
-
Engineered gene circuits.Nature. 2002 Nov 14;420(6912):224-30. doi: 10.1038/nature01257. Nature. 2002. PMID: 12432407 Review.
-
Synthetic gene circuits: design with directed evolution.Annu Rev Biophys Biomol Struct. 2007;36:1-19. doi: 10.1146/annurev.biophys.36.040306.132600. Annu Rev Biophys Biomol Struct. 2007. PMID: 17243895 Review.
-
Dealing with the genetic load in bacterial synthetic biology circuits: convergences with the Ohm's law.Nucleic Acids Res. 2016 Jan 8;44(1):496-507. doi: 10.1093/nar/gkv1280. Epub 2015 Dec 9. Nucleic Acids Res. 2016. PMID: 26656950 Free PMC article.
-
Introduction to focus issue: quantitative approaches to genetic networks.Chaos. 2013 Jun;23(2):025001. doi: 10.1063/1.4810923. Chaos. 2013. PMID: 23822498
-
Synthetic Gene Expression Circuits for Designing Precision Tools in Oncology.Front Cell Dev Biol. 2017 Aug 28;5:77. doi: 10.3389/fcell.2017.00077. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28894736 Free PMC article. Review.
Cited by
-
Growth Feedback Confers Cooperativity in Resource-Competing Synthetic Gene Circuits.Chaos Solitons Fractals. 2023 Aug;173:113713. doi: 10.1016/j.chaos.2023.113713. Epub 2023 Jun 24. Chaos Solitons Fractals. 2023. PMID: 37485435 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

