Purinergic System Signaling in Metainflammation-Associated Osteoarthritis
- PMID: 32984382
- PMCID: PMC7485330
- DOI: 10.3389/fmed.2020.00506
Purinergic System Signaling in Metainflammation-Associated Osteoarthritis
Abstract
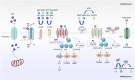
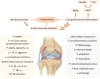
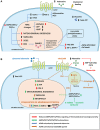
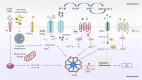
Inflammation triggered by metabolic imbalance, also called metainflammation, is low-grade inflammation caused by the components involved in metabolic syndrome (MetS), including central obesity and impaired glucose tolerance. This phenomenon is mainly due to excess nutrients and energy, and it contributes to the pathogenesis of osteoarthritis (OA). OA is characterized by the progressive degeneration of articular cartilage, which suffers erosion and progressively becomes thinner. Purinergic signaling is involved in several physiological and pathological processes, such as cell proliferation in development and tissue regeneration, neurotransmission and inflammation. Adenosine and ATP receptors, and other members of the signaling pathway, such as AMP-activated protein kinase (AMPK), are involved in obesity, type 2 diabetes (T2D) and OA progression. In this review, we focus on purinergic regulation in osteoarthritic cartilage and how different components of MetS, such as obesity and T2D, modulate the purinergic system in OA. In that regard, we describe the critical role in this disease of receptors, such as adenosine A2A receptor (A2AR) and ATP P2X7 receptor. Finally, we also assess how nucleotides regulate the inflammasome in OA.
Keywords: A2AR; Inflammasome; P2X7 receptor; metainflammation; mitochondrial metabolism; osteoarthritis; purinergic system; rheumatic diseases.
Copyright © 2020 Gratal, Lamuedra, Medina, Bermejo-Álvarez, Largo, Herrero-Beaumont and Mediero.
Figures
Similar articles
-
Purinergic signaling in retinal degeneration and regeneration.Neuropharmacology. 2016 May;104:194-211. doi: 10.1016/j.neuropharm.2015.05.005. Epub 2015 May 19. Neuropharmacology. 2016. PMID: 25998275 Review.
-
Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages.Clin Exp Pharmacol Physiol. 2014 Apr;41(4):279-86. doi: 10.1111/1440-1681.12214. Clin Exp Pharmacol Physiol. 2014. PMID: 24472059
-
Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression.Nat Commun. 2017 May 11;8:15019. doi: 10.1038/ncomms15019. Nat Commun. 2017. PMID: 28492224 Free PMC article.
-
Obesity, Metabolic Syndrome, and Osteoarthritis-An Updated Review.Curr Obes Rep. 2023 Sep;12(3):308-331. doi: 10.1007/s13679-023-00520-5. Epub 2023 Aug 14. Curr Obes Rep. 2023. PMID: 37578613 Review.
-
Inflammation and intracellular metabolism: new targets in OA.Osteoarthritis Cartilage. 2015 Nov;23(11):1835-42. doi: 10.1016/j.joca.2014.12.016. Osteoarthritis Cartilage. 2015. PMID: 26521729 Free PMC article. Review.
Cited by
-
TNAP as a New Player in Chronic Inflammatory Conditions and Metabolism.Int J Mol Sci. 2021 Jan 18;22(2):919. doi: 10.3390/ijms22020919. Int J Mol Sci. 2021. PMID: 33477631 Free PMC article. Review.
-
Stage related metabolic profile of the synovial fluid in patients with acute flares of knee osteoarthritis.Med Pharm Rep. 2022 Oct;95(4):438-445. doi: 10.15386/mpr-2454. Epub 2022 Oct 27. Med Pharm Rep. 2022. PMID: 36506601 Free PMC article.
-
Targeting inflammasome-dependent mechanisms as an emerging pharmacological approach for osteoarthritis therapy.iScience. 2022 Nov 11;25(12):105548. doi: 10.1016/j.isci.2022.105548. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465135 Free PMC article. Review.
-
P2X7Rs: new therapeutic targets for osteoporosis.Purinergic Signal. 2023 Mar;19(1):207-219. doi: 10.1007/s11302-021-09836-0. Epub 2022 Feb 2. Purinergic Signal. 2023. PMID: 35106736 Free PMC article. Review.
-
Systemic osteoarthritis: the difficulty of categorically naming a continuous condition.Aging Clin Exp Res. 2024 Feb 20;36(1):45. doi: 10.1007/s40520-024-02714-w. Aging Clin Exp Res. 2024. PMID: 38376694 Free PMC article. Review.
References
-
- Burnstock G. Purinergic nerves. Pharmacol Rev. (1972) 24:509–81. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

