A Density Functional Theory Study toward Ring-Opening Reaction Mechanisms of 2,4,6-Trinitrotoluene's Meisenheimer Complex for the Biodegradation of Old Yellow Enzyme Flavoprotein Reductase
- PMID: 32984681
- PMCID: PMC7512433
- DOI: 10.1021/acsomega.0c02162
A Density Functional Theory Study toward Ring-Opening Reaction Mechanisms of 2,4,6-Trinitrotoluene's Meisenheimer Complex for the Biodegradation of Old Yellow Enzyme Flavoprotein Reductase
Abstract
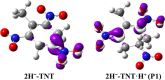
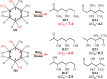
The subsequent degradation pathway of the dihydride-Meisenheimer complex (2H--TNT), which is the metabolite of 2,4,6-trinitrotoluene (TNT) by old yellow enzyme flavoprotein reductases of yeast and bacteria, was investigated computationally at the SMD/TPSSH/6-311+G(d,p) level of theory. Combining the experimentally detected products, a series of protonation, addition, substitution (dearomatization), and ring-opening reaction processes from 2H--TNT to alkanes were proposed. By analyzing reaction free energies, we determined that the protonation is more advantageous thermodynamically than the dimerization reaction. In the ring-opening reaction of naphthenic products, the water molecule-mediated proton transfer mechanism plays a key role. The corresponding activation energy barrier is 37.7 kcal·mol-1, which implies the difficulty of this reaction. Based on our calculations, we gave an optimum pathway for TNT mineralization. Our conclusions agree qualitatively with available experimental results. The details on transition states, intermediates, and free energy surfaces for all proposed reactions are given and make up for a lack of experimental knowledge.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

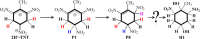




References
-
- Wei T.; Zhou Y.; Yang Z. l.; Yang H. Progress of Toxicity Effects and Mechanisms of Typical Explosives. Chin. J. Energ. Mater. 2019, 27, 558–568.
-
- United States Environmental Protection Agency. Technical fact sheet-2,4,6-trinitrotoluene (TNT). https://www.epa.gov/sites/production/files/2014-03/documents/frrofactsheet contaminant TNT January 2014 final.pdf, 2014.
-
- United States General Accounting Office. Department of Defense operational ranges, more reliable cleanup cost estimates and a proactive approach to identifying contamination are needed. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA435939&Location=U2&doc=GetTRD..., 2004.
LinkOut - more resources
Full Text Sources

