Rational Design of W-Doped Ag3PO4 as an Efficient Antibacterial Agent and Photocatalyst for Organic Pollutant Degradation
- PMID: 32984701
- PMCID: PMC7513369
- DOI: 10.1021/acsomega.0c03019
Rational Design of W-Doped Ag3PO4 as an Efficient Antibacterial Agent and Photocatalyst for Organic Pollutant Degradation
Abstract
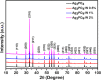
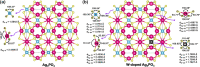
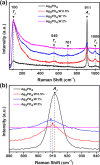
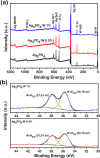
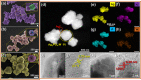
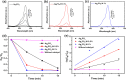
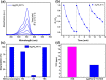
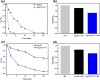
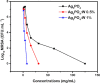
Bacterial and organic pollutants are major problems with potential adverse impacts on human health and the environment. A promising strategy to alleviate these impacts consists in designing innovative photocatalysts with a wider spectrum of application. In this paper, we report the improved photocatalytic and antibacterial activities of chemically precipitated Ag3PO4 microcrystals by the incorporation of W at doping levels 0.5, 1, and 2 mol %. The presence of W directly influences the crystallization of Ag3PO4, affecting the morphology, particle size, and surface area of the microcrystals. Also, the characterization via experimental and theoretical approaches evidenced a high density of disordered [AgO4], [PO4], and [WO4] structural clusters due to the substitution of P5+ by W6+ into the Ag3PO4 lattice. This leads to new defect-related energy states, which decreases the band gap energy of the materials (from 2.27 to 2.04 eV) and delays the recombination of e'-h• pairs, leading to an enhanced degradation process. As a result of such behaviors, W-doped Ag3PO4 (Ag3PO4:W) is a better visible-light photocatalyst than Ag3PO4, demonstrated here by the photodegradation of potential environmental pollutants. The degradation of rhodamine B dye was 100% in 4 min for Ag3PO4:W 1%, and for Ag3PO4, the obtained result was 90% of degradation in 15 min of reaction. Ag3PO4:W 1% allowed the total degradation of cephalexin antibiotic in only 4 min, whereas pure Ag3PO4 took 20 min to achieve the same result. For the degradation of imidacloprid insecticide, Ag3PO4:W 1% allowed 90% of degradation, whereas Ag3PO4 allowed 40%, both in 20 min of reaction. Moreover, the presence of W-dopant results in a 16-fold improvement of bactericidal performance against methicillin-resistant Staphylococcus aureus. The outstanding results using the Ag3PO4:W material demonstrated its potential multifunctionality for the control of organic pollutants and bacteria in environmental applications.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Zhang S.; Li B.; Wang X.; Zhao G.; Hu B.; Lu Z.; Wen T.; Chen J.; Wang X. Recent Developments of Two-dimensional Graphene-based Composites in Visible-light Photocatalysis for Eliminating Persistent Organic Pollutants from Wastewater. Chem. Eng. J. 2020, 390, 12464210.1016/j.cej.2020.124642. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

