Programming Multifaceted Pulmonary T Cell Immunity by Combination Adjuvants
- PMID: 32984856
- PMCID: PMC7508055
- DOI: 10.1016/j.xcrm.2020.100095
Programming Multifaceted Pulmonary T Cell Immunity by Combination Adjuvants
Abstract
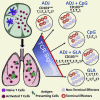
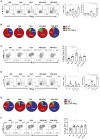
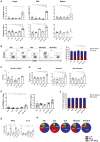
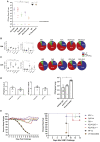
Induction of protective mucosal T cell memory remains a formidable challenge to vaccinologists. Using a combination adjuvant strategy that elicits potent CD8 and CD4 T cell responses, we define the tenets of vaccine-induced pulmonary T cell immunity. An acrylic-acid-based adjuvant (ADJ), in combination with Toll-like receptor (TLR) agonists glucopyranosyl lipid adjuvant (GLA) or CpG, promotes mucosal imprinting but engages distinct transcription programs to drive different degrees of terminal differentiation and disparate polarization of TH1/TC1/TH17/TC17 effector/memory T cells. Combination of ADJ with GLA, but not CpG, dampens T cell receptor (TCR) signaling, mitigates terminal differentiation of effectors, and enhances the development of CD4 and CD8 TRM cells that protect against H1N1 and H5N1 influenza viruses. Mechanistically, vaccine-elicited CD4 T cells play a vital role in optimal programming of CD8 TRM and viral control. Taken together, these findings provide further insights into vaccine-induced multifaceted mucosal T cell immunity with implications in the development of vaccines against respiratorypathogens, including influenza virus and SARS-CoV-2.
Keywords: CD4; CD8; T1 and T17 programming; adjuvants; heterosubtypic; influenza virus; mucosal subunit vaccines; respiratory immunity; tissue-resident memory.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

