Atorvastatin combined with low-dose dexamethasone for vascular endothelial cell dysfunction induced by chronic subdural hematoma
- PMID: 32985481
- PMCID: PMC7996011
- DOI: 10.4103/1673-5374.293152
Atorvastatin combined with low-dose dexamethasone for vascular endothelial cell dysfunction induced by chronic subdural hematoma
Abstract
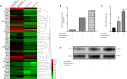
Atorvastatin has been shown to be a safe and effective non-surgical treatment option for patients with chronic subdural hematoma. However, treatment with atorvastatin is not effective in some patients, who must undergo further surgical treatment. Dexamethasone has anti-inflammatory and immunomodulatory effects, and low dosages are safe and effective for the treatment of many diseases, such as ankylosing spondylitis and community-acquired pneumonia. However, the effects of atorvastatin and low-dose dexamethasone for the treatment of chronic subdural hematoma remain poorly understood. Hematoma samples of patients with chronic subdural hematoma admitted to the General Hospital of Tianjin Medical University of China were collected and diluted in endothelial cell medium at 1:1 as the hematoma group. Atorvastatin, dexamethasone, or their combination was added to the culture medium. The main results were as follows: hopping probe ion conductance microscopy and permeability detection revealed that the best dosages to improve endothelial cell permeability were 0.1 μM atorvastatin and 0.1 μM dexamethasone. Atorvastatin, dexamethasone, or their combination could markedly improve the recovery of injured endothelial cells. Mice subcutaneously injected with diluted hematoma solution and then treated with atorvastatin, dexamethasone, or their combination exhibited varying levels of rescue of endothelial cell function. Hopping probe ion conductance microscopy, western blot assay, and polymerase chain reaction to evaluate the status of human cerebral endothelial cell status and expression level of tight junction protein indicated that atorvastatin, dexamethasone, or their combination could reduce subcutaneous vascular leakage caused by hematoma fluid. Moreover, the curative effect of the combined treatment was significantly better than that of either single treatment. Expression of Krüppel-like factor 2 protein in human cerebral endothelial cells was significantly increased, as was expression of the tight junction protein and vascular permeability marker vascular endothelial cadherin in each treatment group compared with the hematoma stimulation group. Hematoma fluid in patients with chronic subdural hematoma may damage vascular endothelial cells. However, atorvastatin combined with low-dose dexamethasone could rescue endothelial cell dysfunction by increasing the expression of tight junction proteins after hematoma injury. The effect of combining atorvastatin with low-dose dexamethasone was better than that of atorvastatin alone. Increased expression of Krüppel-like factor 2 may play an important role in the treatment of chronic subdural hematoma. The animal protocols were approved by the Animal Care and Use Committee of Tianjin Medical University of China on July 31, 2016 (approval No. IRB2016-YX-036). The study regarding human hematoma samples was approved by the Ethics Committee of Tianjin Medical University of China on July 31, 2018 (approval No. IRB2018-088-01).
Keywords: brain; brain trauma; cells; central nervous system; inflammation; plasticity; protein; repair.
Conflict of interest statement
authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures




References
-
- Adelborg K, Grove EL, Sundboll J, Laursen M, Schmidt M. Sixteen-year nationwide trends in antithrombotic drug use in Denmark and its correlation with landmark studies. Heart. 2016;102:1883–1889. - PubMed
-
- Apfelbaum RI, Guthkelch AN, Shulman K. Experimental production of subdural hematomas. J Neurosurg. 1974;40:336–346. - PubMed
-
- Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM. Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012;70:873–880. - PubMed
-
- Bhattacharya R, Senbanerjee S, Lin Z, Mir S, Hamik A, Wang P, Mukherjee P, Mukhopadhyay D, Jain MK. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J Biol Chem. 2005;280:28848–28851. - PubMed
-
- Caporali A, Pani E, Horrevoets AJ, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. 2008;103:e15–e26. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

