Human 2-oxoglutarate-dependent oxygenases: nutrient sensors, stress responders, and disease mediators
- PMID: 32985654
- PMCID: PMC7609023
- DOI: 10.1042/BST20190333
Human 2-oxoglutarate-dependent oxygenases: nutrient sensors, stress responders, and disease mediators
Abstract
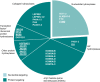
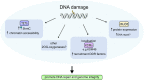
Fe(II)/2-oxoglutarate (2OG)-dependent oxygenases are a conserved enzyme class that catalyse diverse oxidative reactions across nature. In humans, these enzymes hydroxylate a broad range of biological substrates including DNA, RNA, proteins and some metabolic intermediates. Correspondingly, members of the 2OG-dependent oxygenase superfamily have been linked to fundamental biological processes, and found dysregulated in numerous human diseases. Such findings have stimulated efforts to understand both the biochemical activities and cellular functions of these enzymes, as many have been poorly studied. In this review, we focus on human 2OG-dependent oxygenases catalysing the hydroxylation of protein and polynucleotide substrates. We discuss their modulation by changes in the cellular microenvironment, particularly with respect to oxygen, iron, 2OG and the effects of oncometabolites. We also describe emerging evidence that these enzymes are responsive to cellular stresses including hypoxia and DNA damage. Moreover, we examine how dysregulation of 2OG-dependent oxygenases is associated with human disease, and the apparent paradoxical role for some of these enzymes during cancer development. Finally, we discuss some of the challenges associated with assigning biochemical activities and cellular functions to 2OG-dependent oxygenases.
Keywords: disease; hydroxylation; hypoxia; nutrient sensing; oxygenase; post translational modification.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

