Indomethacin can induce cell death in rat gastric parietal cells through alteration of some apoptosis- and autophagy-associated molecules
- PMID: 32985762
- PMCID: PMC7691216
- DOI: 10.1111/iep.12370
Indomethacin can induce cell death in rat gastric parietal cells through alteration of some apoptosis- and autophagy-associated molecules
Abstract
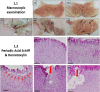
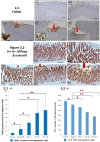
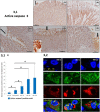
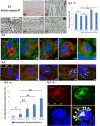
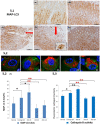
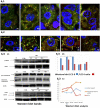
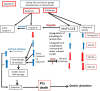
In clinical medicine, indomethacin (IND, a non-steroidal anti-inflammatory drug) is used variously in the treatment of severe osteoarthritis, rheumatoid arthritis, gouty arthritis or ankylosing spondylitis. A common complication found alongside the therapeutic characteristics is gastric mucosal damage. This complication is mediated through apoptosis and autophagy of the gastrointestinal mucosal epithelium. Apoptosis and autophagy are critical homeostatic pathways catalysed by caspases downstream of the gastrointestinal mucosal epithelial injury. Both act through molecular signalling pathways characterized by the initiation, mediation, execution and regulation of the cell regulatory cycle. In this study we hypothesized that dysregulated apoptosis and autophagy are associated with IND-induced gastric damage. We examined the spectra of in vivo experimental gastric ulcers in male Sprague-Dawley rats through gastric gavage of IND. Following an 18-hour fast, IND was administered to experimental rats. They were sacrificed at 3-, 6- and 12-hour intervals. Parietal cells (H+ , K+ -ATPase β-subunit assay) and apoptosis (TUNEL assay) were determined. The expression of apoptosis-signalling caspase (caspases 3, 8, 9 and 12), DNA damage (anti-phospho-histone H2A.X) and autophagy (MAP-LC3, LAMP-1 and cathepsin B)-related molecules in gastric mucosal cells was examined. The administration of IND was associated with gastric mucosal erosions and ulcerations mainly involving the gastric parietal cells (PCs) of the isthmic and upper neck regions and a time-dependent gradual increase in the number of apoptotic PCs with the induction of both apoptotic (upregulation of caspases 3 and 8) cell death and autophagic (MAP-LC3-II, LAMP-1 and cathepsin B) cell death. Autophagy induced by fasting and IND 3 hours initially prompted the degradation of caspase 8. After 6 and 12 hours, damping down of autophagic activity occurred, resulting in the upregulation of active caspase 8 and its nuclear translocation. In conclusion we report that IND can induce time-dependent apoptotic and autophagic cell death of PCs. Our study provides the first indication of the interactions between these two homeostatic pathways in this context.
Keywords: apoptosis; autophagy; gastric mucosa; indomethacin; parietal cells.
© 2020 Company of the International Journal of Experimental Pathology (CIJEP).
Conflict of interest statement
There were no conflicts of interest.
Figures
Similar articles
-
Ultra-structural study of the indomethacin-induced apoptosis and autophagy in rat gastric parietal cells.Ultrastruct Pathol. 2020 May 3;44(3):300-313. doi: 10.1080/01913123.2020.1772429. Epub 2020 Jul 16. Ultrastruct Pathol. 2020. PMID: 32672114
-
Mechanistic insights into the protective effects of chlorogenic acid against indomethacin-induced gastric ulcer in rats: Modulation of the cross talk between autophagy and apoptosis signaling.Life Sci. 2021 Jun 15;275:119370. doi: 10.1016/j.lfs.2021.119370. Epub 2021 Mar 17. Life Sci. 2021. PMID: 33744322
-
Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells.Proc Soc Exp Biol Med. 2000 Jun;224(2):102-8. doi: 10.1046/j.1525-1373.2000.22407.x. Proc Soc Exp Biol Med. 2000. PMID: 10806417
-
Mechanism of non-steroidal anti-inflammatory drug-gastropathy.Dig Liver Dis. 2001 Dec;33 Suppl 2:S35-43. doi: 10.1016/s1590-8658(01)80157-2. Dig Liver Dis. 2001. PMID: 11827361 Review.
-
Apoptosis in the gastric mucosa: molecular mechanisms, basic and clinical implications.J Physiol Pharmacol. 2000 Mar;51(1):3-15. J Physiol Pharmacol. 2000. PMID: 10768847 Review.
Cited by
-
Protective effect of astaxanthin on indomethacin-induced gastric ulcerations in mice.Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9897-9907. doi: 10.1007/s00210-024-03206-4. Epub 2024 Jun 28. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38940848 Free PMC article.
-
Prophylaxis of posterior capsule opacification through autophagy activation with indomethacin-eluting intraocular lens.Bioact Mater. 2022 Dec 6;23:539-550. doi: 10.1016/j.bioactmat.2022.11.024. eCollection 2023 May. Bioact Mater. 2022. PMID: 36514385 Free PMC article.
-
Gastroprotective Effects of Periplaneta americana L. Extract Against Ethanol-Induced Gastric Ulcer in Mice by Suppressing Apoptosis-Related Pathways.Front Pharmacol. 2021 Dec 15;12:798421. doi: 10.3389/fphar.2021.798421. eCollection 2021. Front Pharmacol. 2021. PMID: 34975497 Free PMC article.
-
The Potential Preventive and Therapeutic Roles of NSAIDs in Prostate Cancer.Cancers (Basel). 2023 Nov 16;15(22):5435. doi: 10.3390/cancers15225435. Cancers (Basel). 2023. PMID: 38001694 Free PMC article. Review.
-
Protective effect of valsartan alone and in combination with neprilysin inhibitor (valsartan + sacubitril) against hepatic ischemia-reperfusion injury: targeting angiotensin II receptor-neprilysin and modulating SMAD-4/NF-κβ/JNK pathways in rats.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):8797-8811. doi: 10.1007/s00210-025-03820-w. Epub 2025 Jan 27. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39869188
References
-
- Aoyama F, Sawaguchi A. Functional transformation of gastric parietal cells and intracellular trafficking of ion channels/transporters in the apical canalicular membrane associated with acid secretion. Biol Pharm Bull. 2011;34:813–816. - PubMed
-
- Aoyama F, Sawaguchi A, Ide S, Kitamura K, Suganuma T. Exfoliation of gastric pit‐parietal cells into the gastric lumen associated with a stimulation of isolated rat gastric mucosa in vitro: a morphological study by the application of cryotechniques. Histochem Cell Biol. 2008;129:785–793. - PubMed
-
- Benchoua A, Couriaud C, Guegan C, et al. Active caspase‐8 translocates into the nucleus of apoptotic cells to inactivate poly(ADP‐ribose) polymerase‐2. J Biol Chem. 2002;277:34217–34222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

